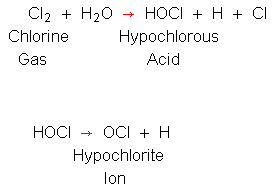

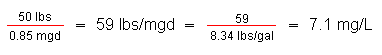
| Chlorine dose in mg/L | Chlorine residual in mg/L | Chlorine demand in mg/L |
| 7.1 | 0.5 | 6.6 |
| Effect of Exposure | Parts of Chlorine Gas Per Million of Air By Volume (ppm) |
| Slight symptoms after several hours exposure | 1 |
| Irritates throat | 10 - 15 |
| Causes coughing | 30 |
| Dangerous in 30 minutes | 40 - 60 |
| Fatal in a few breaths | 1,000 |




