|
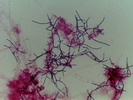
Table 1. Filament Types as Indicators of Conditions Causing Activated Sludge Bulking
|
| Causative Condition |
Filament Types |
| Low D.O. ( For the applied organic loading ) |
S.natans, Type 1701 and H.hydrossis |
| Low Organic Loading Rate ( Low F:M ) |
M.parvicella, Nocardia species, Type 0041, Type 0675,
Type 1851 and Type 0803 |
| Septic Wastes / Sulphides ( High organic acids ) |
Thiothrix I and II, Beggiatoa species, N. limicola II,
Type 021N, Type 0092, Type 0914, Type 0581, Type 0961 and Type 0411 |
| Nutrient Deficiency ( N and / or P ) ( Industrial Wastes Only ) |
For N : Thiothrix I and II and Type 021N
For P : N. limicola III |
| High Grease / Oil |
Nocardia species, M.parvicella and Type 1863 |
| Low pH ( Below pH 6.0 ) |
Fungi |
 Click here for the site...
Click here for the site...
 Click here for the site...
Click here for the site...


 Click here for the site...
Click here for the site...