

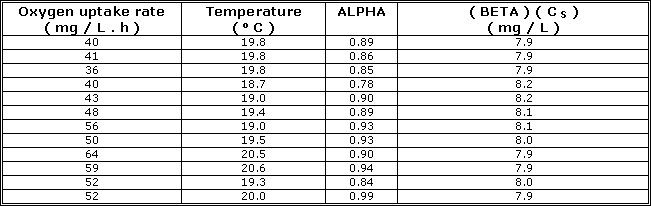
| Temperature ( O C ) | C S ( mg / L ) |
| 0 | 14.62 |
| 2 | 13.84 |
| 4 | 13.13 |
| 6 | 12.48 |
| 8 | 11.87 |
| 10 | 11.33 |
| 12 | 10.83 |
| 14 | 10.37 |
| 16 | 9.95 |
| 18 | 9.54 |
| 20 | 9.17 |
| 22 | 8.83 |
| 24 | 8.53 |
| 26 | 8.22 |
| 28 | 7.92 |
| 30 | 7.63 |
| Atmosphere contains 21 % oxygen, chloride concentration = 0.00 mg / L and pressure = 1 atm. | |

| Temperature ( O C ) | Vapor pressure ( mm Hg ) |
| 0 | 4.5 |
| 5 | 6.5 |
| 10 | 9.2 |
| 15 | 12.8 |
| 20 | 17.5 |
| 25 | 23.8 |
| 30 | 31.8 |













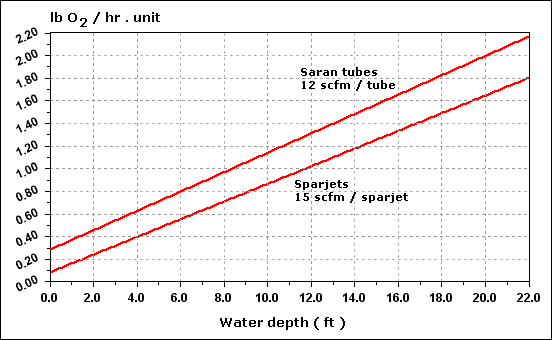

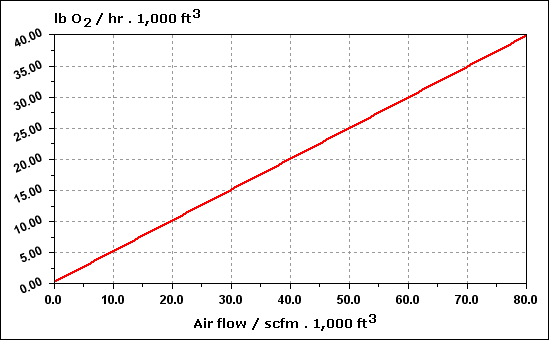
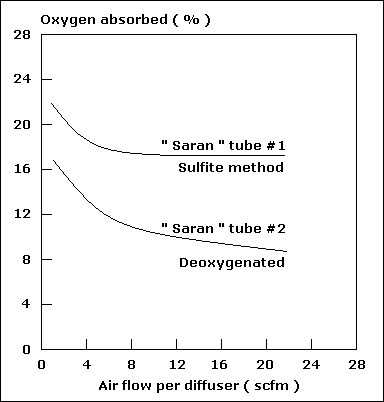
| Temperature ( O C ) |
GAMMA W ( lb / ft 3 ) |
| 0.0 | 62.42 |
| 4.4 | 62.43 |
| 10.0 | 62.41 |
| 15.5 | 62.37 |
| 21.1 | 62.30 |
| 26.6 | 62.22 |
| 32.2 | 62.11 |
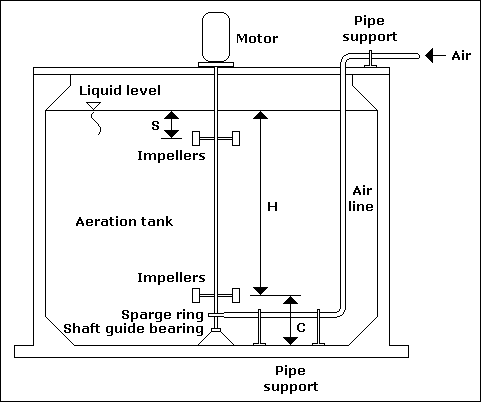
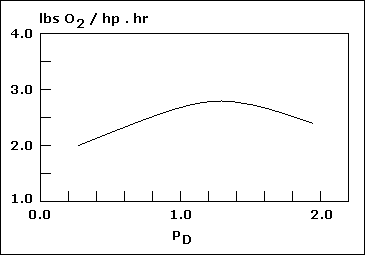
 High - speed aerator... |
 Low - speed aerator... |
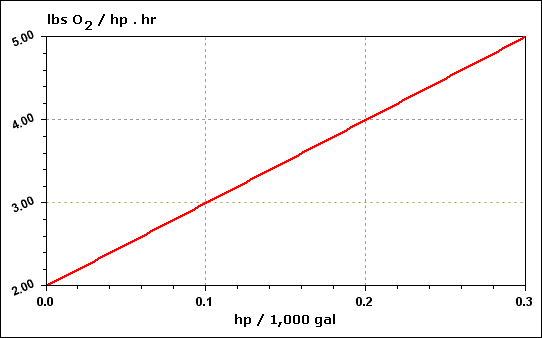
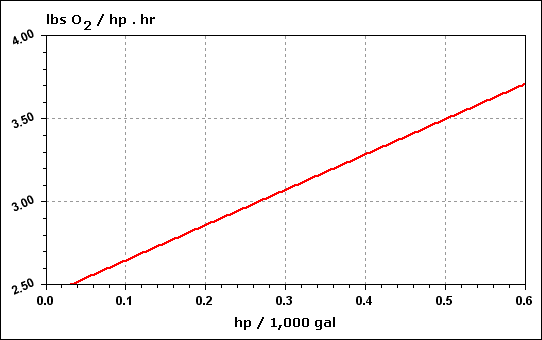
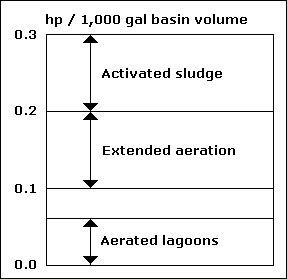
| Hp |
Transfer rate at standard conditions ( lb O 2 / hp . hr ) |
Zone of complete mixing ( ft ) |
Depth ( ft ) |
Zone of complete oxygen dispersion ( ft ) |
Pumping rate through unit ( gpm ) |
| 20 | 3.2 | 72 | 10 | 230 | 8,320 |
| 25 | 3.4 | 80 | 10 | 255 | 9,830 |
| 30 | 3.5 | 88 | 10 | 280 | 12,570 |
| 40 | 3.8 | 102 | 10 | 325 | 14,000 |
| 50 | 3.5 | 105 | 12 | 330 | 18,560 |
| 60 | 3.5 | 115 | 12 | 350 | 20,560 |
| 75 | 3.0 | 130 | 12 | 380 | 22,550 |
| 100 | 3.1 | 150 | 12 | 440 | 41,000 |
| 125 | 3.3 | 165 | 12 | 490 | 47,500 |
| 150 | 3.2 | 185 | 12 | 530 | 57,000 |

