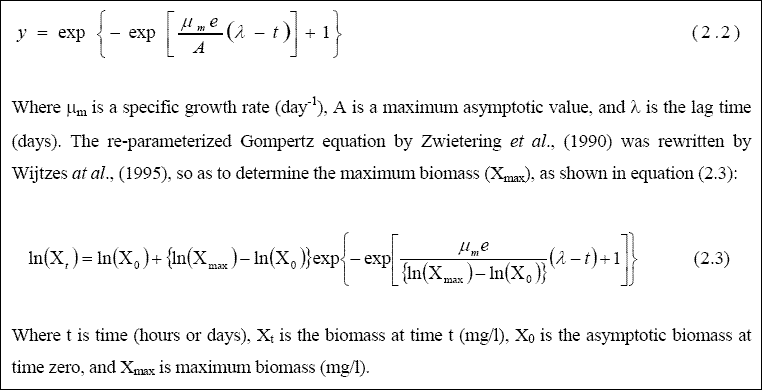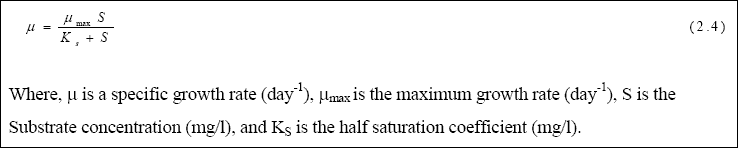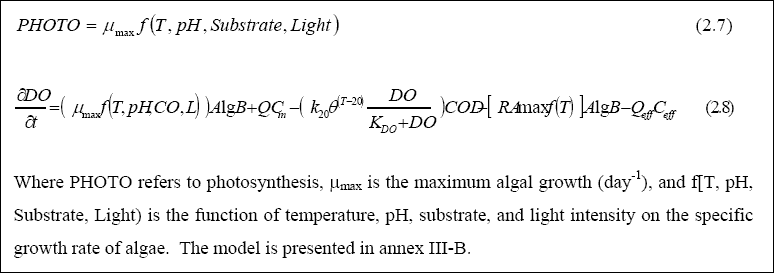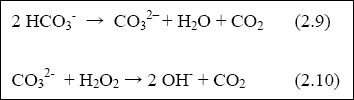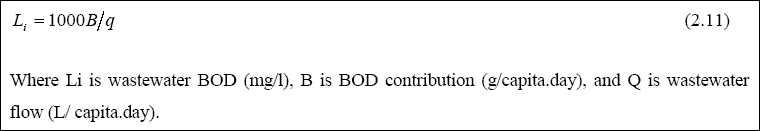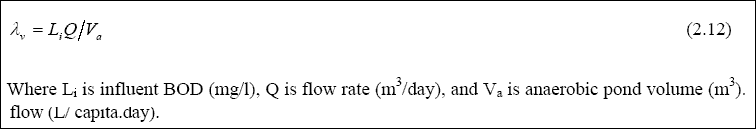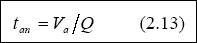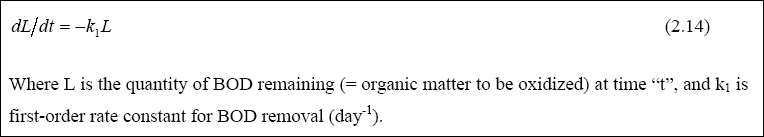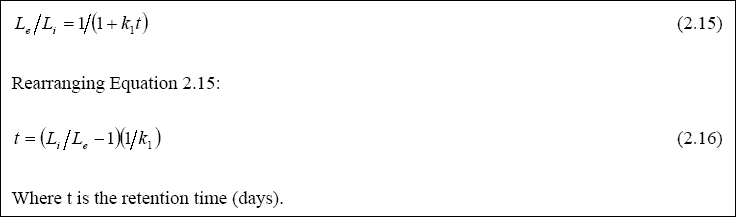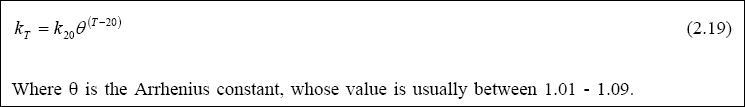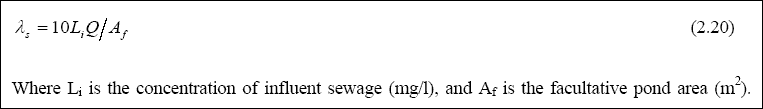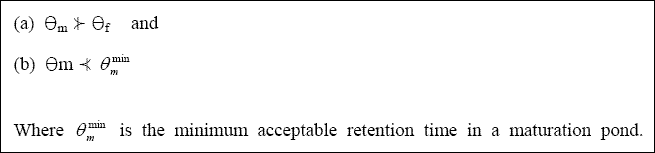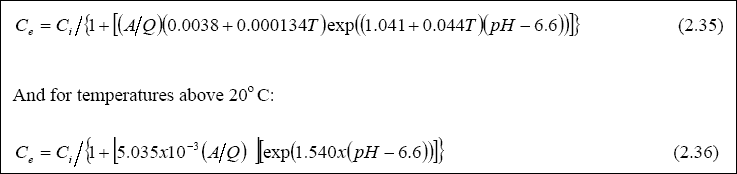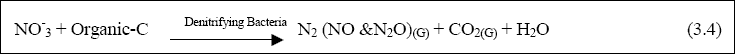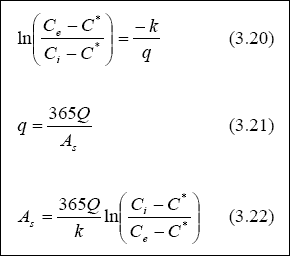Stabilization Ponds - 2...
Introduction...
Importance of Waste Stabilization Ponds and Constructed Wetlands
in Wastewater Treatment in Tropical Climates...
Waste Stabilization Ponds (WSP) and Constructed Wetlands (CW) have proven to be effective alternatives for treating wastewater, and the construction of low energy-consuming ecosystems that use natural processes, in contrast to complex high-maintenance treatment systems, will hopefully lead to more ecologically-sustainable wastewater treatment in the
future. CWs and WSPs also have the capability of meeting the demand for a high percentage removal of pathogenic organisms, compared to conventional technologies. CWs and WSPs combined, and joined with other technologies, may be important for
even more improved performance of water cleaning systems. WSP’s and CW’s are now well-established methods for wastewater treatment in tropical climates. Their many advantages include: simplicity, low cost, low maintenance, low energy
consumption, robustness, and sustainability. While WSPs are most commonly used for treating domestic wastewaters, they are also successfully used for treating industrial wastewater, including water that contains agro-industrial wastes. One of
the potential advantages of using constructed wetlands is that they do not allow mosquitoes to breed (sub-surface flow wetland). The process of designing WSPs and wetlands, and predicting their performance, is improving rapidly as we gain
more experience with these systems. Many countries in tropical climates use WSPs for wastewater treatment (e.g., Tanzania, Kenya, Malawi, Uganda, Zambia, Botswana, Zimbabwe). Many of these systems have been performing below the required standards, due to lack of proper operation and maintenance (Kayombo et al., 1998). Constructed wetlands have not yet received the deserved attention as an alternative method for wastewater treatment.
Overview...
This manual provides comprehensive technical information about the planning, design, construction and operation of WSPs and CWs for a wide range of applications. To understand and design WSPs and CWs requires the involvement of expertise in a variety of fields, including chemistry, hydrology, soil science, plant biology, natural resources, environmental management, ecology, environmental engineering, surveying, and project management.
Why Produce a Manual for Waste Stabilization Ponds and Constructed Wetlands ?...
This manual has been produced to provide necessary information regarding most aspects of the design and implementation of WSPs and CWs. Lack of such information, and an integrated approach, has led to the development of many WSPs and CWs that
are inappropriate, under-performing, or poorly designed or maintained. The reasons for these problems include:
• Lack
of appreciation by many designers of the complex, physical, biological and chemical processes within WSPs and CWs;
• Lack of consistency in design, construction and operation aimed at optimal performance;
• Lack of appropriate design tools and methodologies suitable for local conditions; and
• Changing nature of rapidly-developed technology Although WSPs and CWs are often relatively simple in engineering terms, they are extremely complex ecological systems. Performance of WSPs and CWs relies not only on good design, but also good construction and operation. This design manual
for WSP and CW has adopted the design approaches detailed by D.D. Mara (WSP design approaches), and the design approach
for the constructed wetlands given by Department of Land and Water Conservation, New South Wales (Volume 1 and 2). Part
one of this manual provides a description of the design of WSPs, while Part two provides a detailed description on the
design of CW’s. The management of WSP and CW is discussed in Part three, while Part four focuses on modelling of WSP and
CW ecosystems. Specific design examples are given as appendices in the file, “Design examples.”
Design Tools and Rapidly-Developing Technology...
WSP and CW designers come from many different backgrounds or disciplines, including civil engineering, environmental engineering, microbiology, chemical engineering, soil science, or natural resources management. Many of the WSP and CW
design tools and models have been adopted from countries with temperate climates. Thus, not all models can be transferred
and used in tropical climate countries. For example, the hydrology and climate in tropical African is significantly
different to that of most States in the United States or Europe. Nutrient removal in these countries has a high priority, whereas the removal of pathogenic organisms has a high priority in tropical countries. Most empirical model or design tools developed and used on-site or regionally-specific WSPs or CWs data. Different characteristics, in terms of climate and hydrology, can lead to problems when models are transferred without appropriate modification for local conditions. Further, using simple tools or “rule of thumb” methods, in lieu of appropriate design techniques, often results in malfunctions or a reduced efficiency in the effect of WSP’s and CW’s.
Objectives...
The objectives of this waste stabilization and constructed wetland manual are as follows:
• To provide WSP and CW designers, builders and operators with appropriate information to develop, implement and operate WSP and CW for a range
of applications and design objectives;
• To provide standard systems approach that can be adopted universally, and
which can accommodate a development technology, with changes in information concepts and ideas with time;
• Provide theoretical background on the biological, chemical and physical processes of WSPs and CWs, the current state of the technology and technical knowledge on how to design, operate and maintain the systems; and
• Provide theoretical knowledge on how the models can be used in the best manner to describe the systems.
Waste Stabilization Ponds...
Application of Waste Stabilization Pond Systems...
Waste Stabilization Ponds (WSPs) are large, shallow basins in which raw sewage is treated entirely by natural processes involving both algae and bacteria. They are used for sewage treatment in temperate and tropical climates, and represent one of the most cost-effective, reliable and easily-operated methods for treating domestic and industrial wastewater. Waste stabilization ponds are very effective in the removal of faecal coliform bacteria. Sunlight energy is the only requirement for its operation. Further, it requires minimum supervision for daily operation, by simply cleaning the outlets and inlet works. The temperature and duration of sunlight in tropical countries offer an excellent opportunity for high efficiency
and satisfactory performance for this type of water-cleaning system. They are well-suited for low-income tropical countries where conventional wastewater treatment cannot be achieved due to the lack of a reliable energy source. Further, the advantage of these systems, in terms of removal of pathogens, is one of the most important reasons for its use.
Types of Waste Stabilization Ponds and Their Specific Uses...
WSP systems comprise a single string of anaerobic, facultative and maturation ponds in series, or several such series in parallel. In essence, anaerobic and facultative ponds are designed for removal of Biochemical Oxygen Demand (BOD), and maturation ponds for pathogen removal, although some BOD removal also occurs in maturation ponds and some pathogen removal
in anaerobic and facultative ponds (Mara, 1987). In most cases, only anaerobic and facultative ponds will be needed for BOD removal when the effluent is to be used for restricted crop irrigation and fish pond fertilization, as well as when weak sewage is to be treated prior to its discharge to surface waters. Maturation ponds are only required when the effluent is to be used for unrestricted irrigation, thereby having to comply with the WHO guideline of >1000 faecal coliform bacteria/100 ml. The WSP does not require mechanical mixing, needing only sunlight to supply most of its oxygenation. Its performance may be measured in terms of its removal of BOD and faecal coliform bacteria.
Processes in Waste Stabilization Ponds...
Anaerobic Ponds...
Anaerobic ponds are commonly 2 – 5 m deep and receive wastewater with high organic loads (i.e., usually greater than 100 g BOD / m3 . day, equivalent to more than 3,000 kg / ha . day for a depth of 3 m). They normally do not contain dissolved oxygen or algae. In anaerobic ponds, BOD removal is achieved by sedimentation of solids, and subsequent anaerobic digestion in the resulting sludge. The process of anaerobic digestion is more intense at temperatures above 15O
C. The anaerobic bacteria are usually sensitive to pH < 6.2. Thus, acidic wastewater must be neutralized prior to its treatment in anaerobic ponds. A properly-designed anaerobic pond will achieve about a 40 % removal of BOD at 10O C, and more than 60 % at 20O C. A shorter retention time of 1.0 - 1.5 days is commonly used.
Facultative Ponds...
Facultative ponds (1 - 2 m deep) are of two types: Primary facultative ponds that receive raw wastewater, and secondary facultative ponds that receive particle-free wastewater (usually from anaerobic ponds, septic tanks, primary facultative ponds, and shallow sewerage systems). The process of oxidation of organic matter by aerobic bacteria is usually dominant
in primary facultative ponds or secondary facultative ponds. The processes in anaerobic and secondary facultative ponds
occur simultaneously in primary facultative ponds, as shown in Figure 2.1. It is estimated that about 30 % of the influent BOD leaves the primary facultative pond in the form of methane (Marais 1970). A high proportion of the BOD that does not leave the pond as methane ends up in algae. This process requires more time, more land area, and possibly 2 -3 weeks water retention time, rather than 2 -3 days in the anaerobic pond. In the secondary facultative pond (and the upper layers
of primary facultative ponds), sewage BOD is converted into “Algal BOD,” and has implications for effluent quality requirements. About 70 – 90 % of the BOD of the final effluent from a series of well-designed WSPs is related to the
algae they contain.
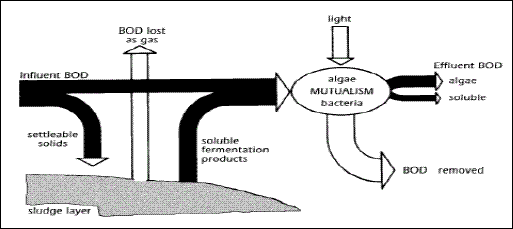
Figure 2.1 Pathways of BOD removal in primary facultative ponds (After Marais, 1970)
In secondary facultative ponds that receive particle-free sewage (anaerobic effluent), the remaining non-settleable BOD is oxidised by heterotrophic bacteria (Pseudomonas, Flavobacterium, Archromobacter and Alcaligenes spp). The oxygen required
for oxidation of BOD is obtained from photosynthetic activity of the micro-algae that grow naturally and profusely in facultative ponds Facultative ponds are designed for BOD removal on the basis of a relatively low surface loading (100 – 400 kg BOD/ha.day), in order to allow for the development of a healthy algal population, since the oxygen for BOD removal by the pond bacteria is generated primarily via algal photosynthesis. The facultative pond relies on naturally-growing algae. The facultative ponds are usually dark-green in colour because of the algae they contain. Motile algae (Chlamydomonas and Euglena) tend to predominate the turbid water in facultative ponds, compared to none-motile algae (Chlorella). The algal concentration in the pond depends on nutrient loading, temperature and sunlight, but is usually in the range of 500 - 2000
µg chlorophyll-a/liter (Mara, 1987). Because of the photosynthetic activities of pond algae, there is a diurnal variation
in the dissolved oxygen concentration. The dissolved oxygen concentration in the water gradually rises after sunrise, in response to photosynthetic activity, to a maximum level in the mid-afternoon, after which it falls to a minimum during the night, when photosynthesis ceases and respiratory activities consume oxygen. At peak algal activity, carbonate and bicarbonate ions react to provide more carbon dioxide for the algae, leaving an excess of hydroxyl ions. As a result, the
pH of the water can rise to above 9, which can kill faecal coliform. Good water mixing, which is usually facilitated by 8
wind within the upper water layer, ensures a uniform distribution of BOD, dissolved oxygen, bacteria and algae, thereby leading to a better degree of waste stabilization.
Kinetic Processes in Facultative Ponds...
The growth of the mixed culture was studied at concentrations ranging between 200 - 800 mg COD/l, in a series of batch chemostat reactors. From laboratory data, the specific growth rate (µ) was determined, using the modified Gompertz model (Kayombo et al. 2003). There are several growth models in the literature used to evaluate growth parameters, examples being the models of Gompertz (1825), Richards (1959), Stannards et al., (1985), Schnute (1981), logistic models, etc. Most of
these models describe the number of microorganisms, without considering the consumption of substrate. Zwietering et al, (1990) derived a modified mathematical relationship based on the Gompertz model for the increase in biomass over time,
which relates the population size over time to the specific growth rate, lag time, and asymptotic level of organisms, as shown in equation (2.2):
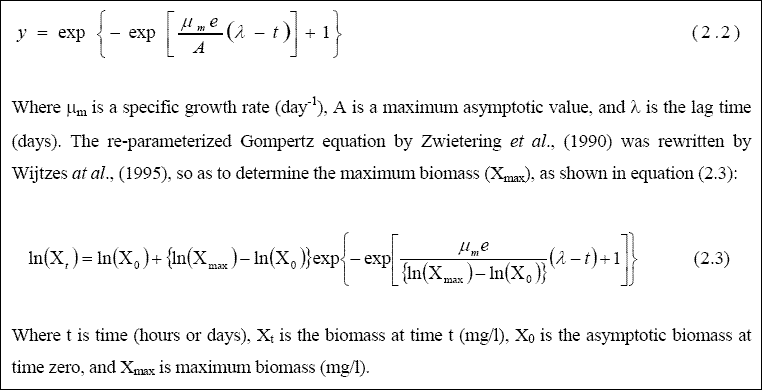
The maximum specific growth rate (µMAX) and half saturation coefficients (KS) were calculated using
the Monod kinetic equation. The Monod (1949) equation is the most commonly used model to relate microbial growth to
substrate utilization. Monod found that, in pure cultures and continuous reactor systems, the relationship between the bacterial growth and substrate availability in the system, with a single growth-limiting substrate, could be expressed empirically, as shown in equation (2.4):
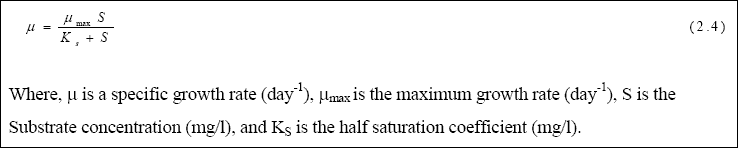
To determine the biological parameters in the Gompertz model, the simulation was done with Stella IIR software, which integrated the model using the built-in 4th Order Runge Kutta. The specific growth rate and lag time (LAMBDA) determined at each concentration of organic carbon, using the Gompertz model, is shown in Table 2.1. The values in the brackets are the correlation coefficients (R2) between the measured and simulated results for specific growth rates of heterotrophic bacteria and algae biomass.
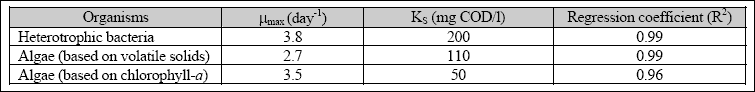
Table 2.1 Specific biomass growth rates
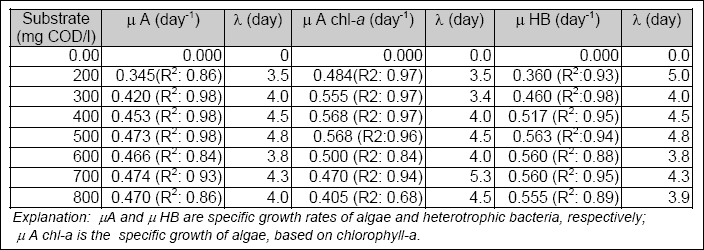 The specific growth rate of algae and heterotrophic bacteria obtained from the modified Gompertz equation was then fit
into the Monod model (i.e., Equation 2.4) to determine the µMAX and KS. The maximum observed growth rate (µMAX) for heterotrophic bacteria was 3.8 day- 1, with a KS of 200 mg COD/l. The µMAX for algal biomass, based on volatile suspended solids, was 2.7 day- 1, with a KS
of 110 mg COD/l. The µMAX of algae, based on chlorophyll-a, was 3.5 day- 1 with a KS of
50 mg COD/l. Table 2.2 summarizes the kinetic constants for processes in secondary facultative ponds.
Table 2.2 Maximum and half-saturation coefficients
The specific growth rate of algae and heterotrophic bacteria obtained from the modified Gompertz equation was then fit
into the Monod model (i.e., Equation 2.4) to determine the µMAX and KS. The maximum observed growth rate (µMAX) for heterotrophic bacteria was 3.8 day- 1, with a KS of 200 mg COD/l. The µMAX for algal biomass, based on volatile suspended solids, was 2.7 day- 1, with a KS
of 110 mg COD/l. The µMAX of algae, based on chlorophyll-a, was 3.5 day- 1 with a KS of
50 mg COD/l. Table 2.2 summarizes the kinetic constants for processes in secondary facultative ponds.
Table 2.2 Maximum and half-saturation coefficients
 The observed specific substrate removal by heterotrophic bacteria varied between the concentrations of substrate used,
with an average value of 0.82 (mg COD/mg biomass). Thus, the determined Monod kinetic parameters are useful for defining
the operation of secondary facultative ponds. The specific substrate utilization rate in the bioreactors was directly proportional to the specific growth rate. Specific substrate removal rate (q) : The substrate utilization rate was determined at a steady-state growth rate for each batch reactor. The observed substrate utilization rate was between
0.68 - 0.97 (mg COD/mg biomass). The average substrate removal rate was 0.82 (mg COD/mg biomass). The maximum substrate utilization rate of 0.97 was achieved at a substrate concentration of 400 mg/l. Thus, the bioreactor yield was 1.03 -
1.47 mg biomass/mg COD. The obtained values compare well to the trend of the specific growth rate obtained from the bioreactors. The substrate utilization rate was directly related to the specific growth rate of heterotrophic bacteria,
as also was shown by Papkov et al., 2000. Although the Monod Equation was developed for the pure culture of bacteria
growing in a single organic substrate, the results from this work indicate the model may be used correctly for a mixed culture of algae and heterotrophic bacteria growing in a heterogeneous-mixed organic carbon. The (KS) obtained
are the characteristic of the wastewaters treated by SFPs, which contains a complex mixture of organic carbon. Further,
it appears that the Monod constants depend on the nature of the operation of the system. The parameters obtained during
this experimental work are applicable for the SFPs where algae and bacteria grow symbiotically. The Monod constants for
algae depend on the method used in determining the biomass. In this research, the reported values are based on the chlorophyll-a and volatile solids values, thus adding more knowledge to our understanding of the processes of facultative ponds.
Effects of pH on the Growth Rate of Heterotrophic Bacteria and Algae
The observed specific substrate removal by heterotrophic bacteria varied between the concentrations of substrate used,
with an average value of 0.82 (mg COD/mg biomass). Thus, the determined Monod kinetic parameters are useful for defining
the operation of secondary facultative ponds. The specific substrate utilization rate in the bioreactors was directly proportional to the specific growth rate. Specific substrate removal rate (q) : The substrate utilization rate was determined at a steady-state growth rate for each batch reactor. The observed substrate utilization rate was between
0.68 - 0.97 (mg COD/mg biomass). The average substrate removal rate was 0.82 (mg COD/mg biomass). The maximum substrate utilization rate of 0.97 was achieved at a substrate concentration of 400 mg/l. Thus, the bioreactor yield was 1.03 -
1.47 mg biomass/mg COD. The obtained values compare well to the trend of the specific growth rate obtained from the bioreactors. The substrate utilization rate was directly related to the specific growth rate of heterotrophic bacteria,
as also was shown by Papkov et al., 2000. Although the Monod Equation was developed for the pure culture of bacteria
growing in a single organic substrate, the results from this work indicate the model may be used correctly for a mixed culture of algae and heterotrophic bacteria growing in a heterogeneous-mixed organic carbon. The (KS) obtained
are the characteristic of the wastewaters treated by SFPs, which contains a complex mixture of organic carbon. Further,
it appears that the Monod constants depend on the nature of the operation of the system. The parameters obtained during
this experimental work are applicable for the SFPs where algae and bacteria grow symbiotically. The Monod constants for
algae depend on the method used in determining the biomass. In this research, the reported values are based on the chlorophyll-a and volatile solids values, thus adding more knowledge to our understanding of the processes of facultative ponds.
Effects of pH on the Growth Rate of Heterotrophic Bacteria and Algae
in Secondary Facultative Ponds...
Knowledge of the effects of the pH on the growth rate of algae and heterotrophic bacteria, and its subsequent impacts on
the degradation of organic matter, is useful for better operation and design of secondary facultative ponds (SFP). The kinetics of microbial growth and product formations are influenced by diurnal variation in the pH in the pond resulting
from diurnal variations in the carbon dioxide (Fritz et al., (1979). Algae require large quantities of dissolved carbon dioxide during photosynthesis, causing a depletion of carbon dioxide (CO2) and leading to a shift in the
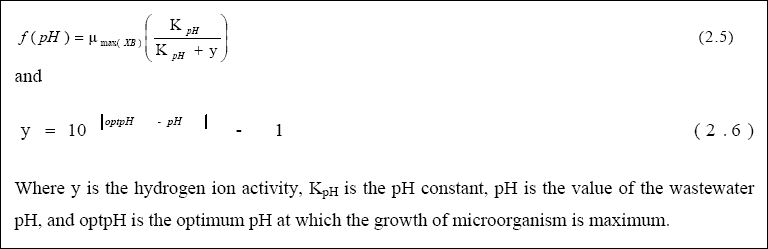
carbonate – bicarbonate (CO3- 2 - HCO3 -) equilibrium, resulting in an increase in pH due to the formation of hydroxyl (OH -) ions (Neel,et al., 1961; Poon et al., 1986; Van der Ploeg, 1982). The effects of pH on the growth rate of heterotrophic bacteria and algae in Secondary Facultative Ponds (SFP) were investigated, using batch growth at a pH value between 5 and 11. The optimum pH and pH constant for algae and heterotrophic bacteria was determined using the pH model presented by Henze et al., (1995):
The determination of the specific growth rate of heterotrophic bacteria and algae under the influence of the pH, based on
the data obtained from the reactors, was done by using the modified Gompertz model presented by Zwietering et al., (1990),
as shown in Equation 2.2. Equation 2.2 was programmed with Stella IIR software. The integration was done with
the built-in 4th Order Runge Kutta. The natural logarithmic values of the measured biomass data were compared
with the predicted specific growth rates by the modified Gompertz model. The observed specific growth rates and other coefficients obtained are as shown in Table 2.3 and 2.4 for heterotrophic bacteria and algae biomass. The linear
regression coefficient (R2) between the measured and model-predicted specific growth rates also is shown
in Table 2.3 and 2.4.
Table 2.3 Specific growth rates for heterotrophic bacteria and algae
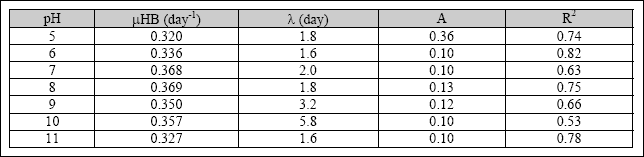 Table 2.4 Specific growth rate for algae
Table 2.4 Specific growth rate for algae
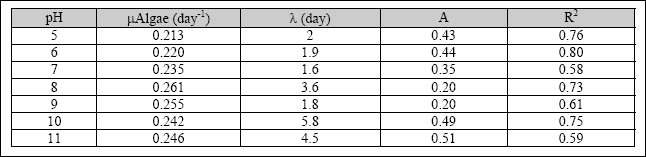 The results indicate that, for a pH value higher than 8, the chlorophyll-a content decreases. However, the specific growth rate of heterotrophic bacteria and algae was high with a pH value between pH 7 to 8. At a pH value above 9, the specific growth rate of both biomasses started to decrease. The optimum pH and pH constant for microbial activities in SFP was determined with the model presented by Henze et al. (1995), as shown in Equations 2.5 and 2.6. The specific growth rate
of microorganisms in the SFP was increasing from a pH value of 5, reaching a maximum value at a pH between 6.5 and 8. The observed optimum pH for heterotrophic bacteria was 8.5, and the pH constant was 200. The optimum pH for algae was 6.8,
and the pH constant was 179 (Kayombo et al., 2001). The parameters were obtained at a maximum growth rate of 3.6
day- 1and 3.45 day- 1 for heterotrophic bacteria and algal biomass, respectively. The region of pH insensitivity for heterotrophic bacteria was between 7 and 9. However, the region of pH insensitivity for algae was between 6.5 and 8. The range between the optimum pH for algae and heterotrophic bacteria was 1.7 pH units. Table 2.5 shows the parameters obtained for the influence of the pH on the growth rate of heterotrophic bacteria and algae.
Table 2.5 The optimum and pH constant for algae and heterotrophic bacteria
The results indicate that, for a pH value higher than 8, the chlorophyll-a content decreases. However, the specific growth rate of heterotrophic bacteria and algae was high with a pH value between pH 7 to 8. At a pH value above 9, the specific growth rate of both biomasses started to decrease. The optimum pH and pH constant for microbial activities in SFP was determined with the model presented by Henze et al. (1995), as shown in Equations 2.5 and 2.6. The specific growth rate
of microorganisms in the SFP was increasing from a pH value of 5, reaching a maximum value at a pH between 6.5 and 8. The observed optimum pH for heterotrophic bacteria was 8.5, and the pH constant was 200. The optimum pH for algae was 6.8,
and the pH constant was 179 (Kayombo et al., 2001). The parameters were obtained at a maximum growth rate of 3.6
day- 1and 3.45 day- 1 for heterotrophic bacteria and algal biomass, respectively. The region of pH insensitivity for heterotrophic bacteria was between 7 and 9. However, the region of pH insensitivity for algae was between 6.5 and 8. The range between the optimum pH for algae and heterotrophic bacteria was 1.7 pH units. Table 2.5 shows the parameters obtained for the influence of the pH on the growth rate of heterotrophic bacteria and algae.
Table 2.5 The optimum and pH constant for algae and heterotrophic bacteria
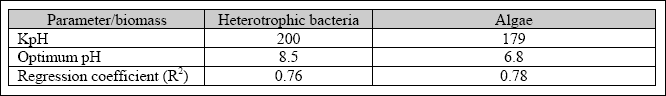 It was concluded that the pH simultaneously affects algae and heterotrophic bacteria processes in facultative ponds with an almost equal magnitude. The model reported by Henze at al., (1995) was found to simulate well the influence of pH on algae and heterotrophic bacteria biomasses.
Diurnal Variation of Dissolved Oxygen in Facultative Waste Stabilization Ponds...
Oxygen tension in WSP’s is an operational parameter with a great deal of daily and hourly variation. The rate of oxygen production is a function of the algal concentration. Because algal growth is both light and temperature - dependent, the
rate of oxygen production (photosynthetic) follows the same pattern. Temperature also is a parameter exhibiting marked seasonal and daily variation in WSP’s. It influences photosynthesis, the growth of microorganisms, and the bio-decomposition of organic carbon in the system. The fluctuation in pH influences the kinetics of microbial growth, species competition and product formations in the pond (Fritz et al., 1979). Microbial species can grow within a specific pH range, which typically extends over 3 to 4 pH units, with an optimum growth rate near the mid-point of the range. Values of pH up to 11 are not uncommon in WSP’s, with the highest levels being reached in the late afternoon. Thus, the combined effects of changes in temperature, pH and light intensity may have a marked effect on microbial activities in the pond, than when only one factor is considered. In reality, changes in these forcing functions and processes occur simultaneously; thus, their influence on the processes should be determined at this level. The objective of the work reported herein was to use the long-term data collected from the ponds to determine the manner in which pH, temperature and light intensity influence the production and utilization of dissolved oxygen in SFWSP. The influence of these forcing functions on the DO model was determined for their combined effect, rather than their effect at the individual level. The dissolved oxygen sub-model was developed in order to depict the combined influences of light, pH, temperature and carbon dioxide on the processes of dissolved oxygen production and utilization in SFWSP. The model was formulated on the basis of the work of Chen and Orlob (1975), being modified to include the influence of pH and carbon dioxide (Kayombo et al., 2000), as follows:
It was concluded that the pH simultaneously affects algae and heterotrophic bacteria processes in facultative ponds with an almost equal magnitude. The model reported by Henze at al., (1995) was found to simulate well the influence of pH on algae and heterotrophic bacteria biomasses.
Diurnal Variation of Dissolved Oxygen in Facultative Waste Stabilization Ponds...
Oxygen tension in WSP’s is an operational parameter with a great deal of daily and hourly variation. The rate of oxygen production is a function of the algal concentration. Because algal growth is both light and temperature - dependent, the
rate of oxygen production (photosynthetic) follows the same pattern. Temperature also is a parameter exhibiting marked seasonal and daily variation in WSP’s. It influences photosynthesis, the growth of microorganisms, and the bio-decomposition of organic carbon in the system. The fluctuation in pH influences the kinetics of microbial growth, species competition and product formations in the pond (Fritz et al., 1979). Microbial species can grow within a specific pH range, which typically extends over 3 to 4 pH units, with an optimum growth rate near the mid-point of the range. Values of pH up to 11 are not uncommon in WSP’s, with the highest levels being reached in the late afternoon. Thus, the combined effects of changes in temperature, pH and light intensity may have a marked effect on microbial activities in the pond, than when only one factor is considered. In reality, changes in these forcing functions and processes occur simultaneously; thus, their influence on the processes should be determined at this level. The objective of the work reported herein was to use the long-term data collected from the ponds to determine the manner in which pH, temperature and light intensity influence the production and utilization of dissolved oxygen in SFWSP. The influence of these forcing functions on the DO model was determined for their combined effect, rather than their effect at the individual level. The dissolved oxygen sub-model was developed in order to depict the combined influences of light, pH, temperature and carbon dioxide on the processes of dissolved oxygen production and utilization in SFWSP. The model was formulated on the basis of the work of Chen and Orlob (1975), being modified to include the influence of pH and carbon dioxide (Kayombo et al., 2000), as follows:
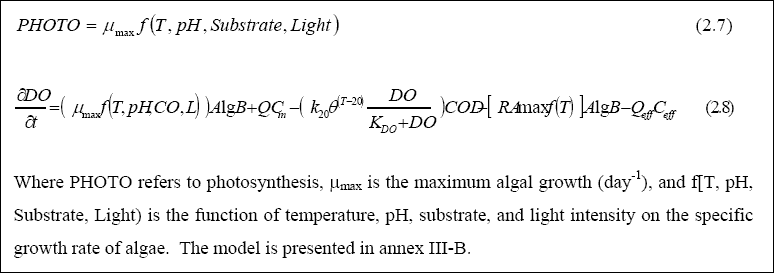
The forcing functions in the dissolved oxygen model were light intensity, carbon dioxide, temperature and pH. It was found that temperature; light and pH influence the photosynthesis process, based on the multiplicative formulation of forcing functions. The model was calibrated and validated by using the average daily data from the Secondary Facultative Waste Stabilization Pond 1 and 11 (SFWSP1 & 11), yielding a linear regression coefficient of 0.87 during calibration, and 0.78 during validation. Based on the model results, the rate of DO production in relation to dry algal biomass was 1.599 mg
DO/mg dry weight (equivalent to 35.905 mg DO/mg chlorophyll-a). This correlation between the observed data and model prediction indicates the assumption inherent in the mathematical model formulation of the processes is valid for describing DO production and usage in the ponds. The developed and validated oxygen sub-model of SFWSP reveals that all forcing functions simultaneously affect the rate of photosynthesis, based on the multiplicative function. It also suggests that,
for a balanced system, the quantity of DO produced by the photosynthesis process is sufficient to keep the system healthy. Based on the model, the major oxygen utilization was related to total respiration. Although the developed model is valid
for SFWSPs, with sufficient data it also may be extended to maturation ponds. The model provides the rate that controls
the oxygen production at which all forcing functions fluctuate simultaneously, a study that might be difficult to conduct
at the laboratory level. The rates obtained during the model calibration and validation are the average governing rates
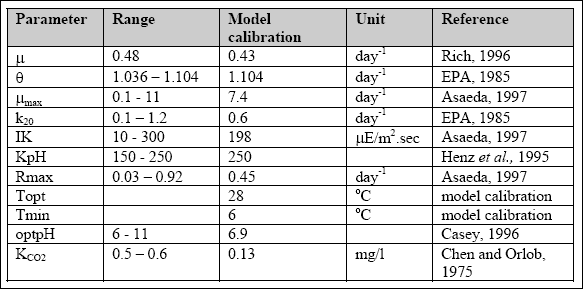
of the performance of the pond under the influence of all forcing functions (Table 2.6).
Table 2.6 The model parameters used and sources of estimation (Kayombo et al., 2000)
 These results lead to the conclusion that the most useful rate that may be used to govern the processes from the WSP should be those determined from the combined influence of the various forcing functions.
Maturation Ponds...
The maturation ponds, usually 1-1.5 m deep, receive the effluent from the facultative ponds. Their primary function is to remove excreted pathogens. Although maturation ponds achieve only a small degree of BOD removal, their contribution to nutrient removal also can be significant. Maturation ponds usually show less vertical biological and physicochemical stratification, and are well-oxygenated throughout the day. The algal population in maturation ponds is much more diverse than that of the facultative ponds, with non-motile genera tending to be more common. The algal diversity generally
increases from pond to pond along the series (Mara, 1989). Although faecal bacteria are partially removed in the
facultative ponds, the size and numbers of the maturation ponds especially determine the numbers of faecal bacteria in the final effluent. There is some removal of solids-associated bacteria in anaerobic ponds, principally by sedimentation. The principal mechanisms for faecal bacterial removal in facultative and maturation ponds are now known to be: (a) Time and temperature; (b) High pH (> 9); and (c) High light intensity, combined with high dissolved oxygen concentration. Time and temperature are the two principal parameters used in designing maturation ponds. Faecal bacterial die-off in ponds increases with both time and temperature (Feachem et al., 1983). High pH values (above 9) occur in ponds, due to rapid photosynthesis by pond algae, which consumes CO2 faster than it can be replaced by bacterial respiration. As a result, carbonate and bicarbonate ions dissociate, as follows:
These results lead to the conclusion that the most useful rate that may be used to govern the processes from the WSP should be those determined from the combined influence of the various forcing functions.
Maturation Ponds...
The maturation ponds, usually 1-1.5 m deep, receive the effluent from the facultative ponds. Their primary function is to remove excreted pathogens. Although maturation ponds achieve only a small degree of BOD removal, their contribution to nutrient removal also can be significant. Maturation ponds usually show less vertical biological and physicochemical stratification, and are well-oxygenated throughout the day. The algal population in maturation ponds is much more diverse than that of the facultative ponds, with non-motile genera tending to be more common. The algal diversity generally
increases from pond to pond along the series (Mara, 1989). Although faecal bacteria are partially removed in the
facultative ponds, the size and numbers of the maturation ponds especially determine the numbers of faecal bacteria in the final effluent. There is some removal of solids-associated bacteria in anaerobic ponds, principally by sedimentation. The principal mechanisms for faecal bacterial removal in facultative and maturation ponds are now known to be: (a) Time and temperature; (b) High pH (> 9); and (c) High light intensity, combined with high dissolved oxygen concentration. Time and temperature are the two principal parameters used in designing maturation ponds. Faecal bacterial die-off in ponds increases with both time and temperature (Feachem et al., 1983). High pH values (above 9) occur in ponds, due to rapid photosynthesis by pond algae, which consumes CO2 faster than it can be replaced by bacterial respiration. As a result, carbonate and bicarbonate ions dissociate, as follows:
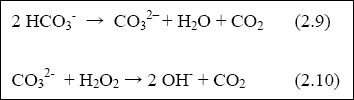
The resulting CO2 is fixed by the algae, and the hydroxyl ions accumulate, often raising the pH to values above 10. Faecal bacteria (with the notable exception of Vibrio cholerae) die very quickly at pH values higher than 9 (Pearson et al., 1987c). The role of high light intensity and high dissolved oxygen concentration has recently been elucidated (Curtis
et al., 1992). Light of wavelengths between 425 – 700 nm can damage faecal bacteria by being absorbed by the humic
substances ubiquitous in wastewater. They remain in an excited state sufficiently long to damage the cell. Light-mediated die-off is completely dependent on the presence of oxygen, as well as being enhanced at high pH values. Thus, the sun plays
a three-fold role in directly promoting faecal bacterial removal in WSP, and in increasing the pond temperature, and more indirectly by providing the energy for rapid algal photosynthesis. This not only raises the pond pH value above 9, but also results in high dissolved oxygen concentrations, which are necessary for its third role; namely, promoting photo-oxidative damage.
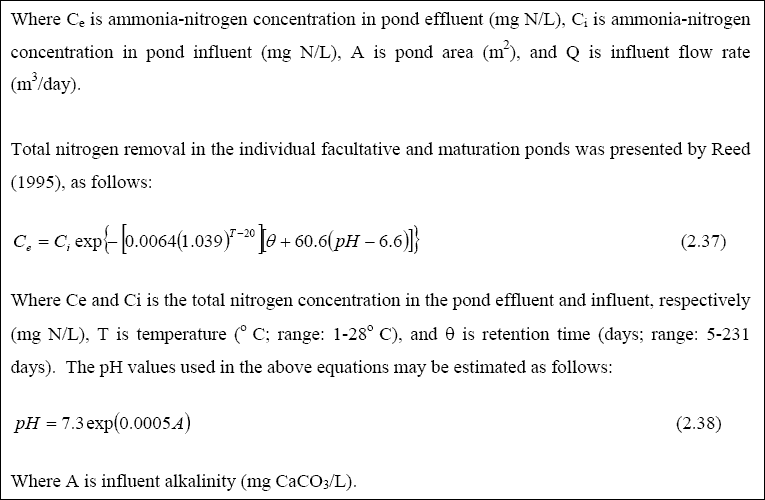
Nutrient Removal in Waste Stabilization Ponds...
In anaerobic ponds, organic nitrogen is hydrolysed to ammonia. Thus, the effluent from anaerobic ponds usually has higher concentrations of ammonia than in raw sewage. In facultative and maturation ponds, ammonia is incorporated into algal biomass. At high pH values, ammonia leaves the pond through volatilization. There is little evidence for nitrification
(and hence denitrification, unless the wastewater has a high nitrate content) (Mara, 1997). This is due to the fact that
the population of the nitrifying bacteria is low because of the lack of physical attachment sites in the aerobic zone.
Total nitrogen and ammonia removal from WSP can reach 80 and 95%, respectively (Mara, 1997). Phosphorus removal in WSP is associated with its uptake by algal biomass, precipitation and sedimentation. Mara (1997) suggested that the best way to remove much of the phosphorus in the wastewater by WSP is to increase the number of maturation ponds. However, both
nitrogen and phosphorus must be removed in order to prevent eutrophication in receiving waterbodies. The common practice
in the design of the WSP is not based on nutrient removal; rather, it is based on BOD and faecal coliform removal.
Design of Waste Stabilization Ponds...
Design Parameters...
There are four important design parameters for WSP, including temperature, net evaporation, flow and BOD. The climate
also is important inasmuch as the processes responsible for BOD5 and fecal bacterial removal are temperature-dependent. Further, algal photosynthesis depends on solar insulation, itself a function of latitude and cloud cover. Cloud cover periods are seldom a problem because the solar insulation during the day in tropical and sub-tropical regions generally greatly exceeds the saturation light intensity of the algae in the ponds. The design temperature usually
is the mean air temperature in the coolest month (or quarter). The pond water is usually 2 - 3O C warmer than
the air temperature in the cool season, with the reverse also being true. Because the bacteria responsible for treatment
are mesophilic, high temperatures are not a problem. However, low temperatures can be since they slow down the treatment process. In the case of the methanogenic bacteria (crucial to anaerobic digestion), methane production virtually ceases
below temperatures of 15O C. Thus, in areas where the pond temperature remains below 15O C for more than a couple of months of the year, careful consideration should be given to deciding whether or not anaerobic units are needed. Net evaporation (evaporation minus rainfall) must be taken into account during the design of facultative and maturation ponds, but not for anaerobic ponds (Arthur, 1976). Anaerobic ponds generally have a scum layer, which
effectively prevents significant evaporation. Total nitrogen and free ammonia (NH3, rather than
NH4+ + NH3) are important in the design of wastewater-fed fishponds. Typical
concentrations of total nitrogen in raw domestic wastewater are 20-70 mg N/l, and total ammonia (NH4+
+ NH3) concentrations are 15 – 40 mg N/l. Faecal coliform numbers are important if the pond effluent is to be
used for unrestricted crop irrigation or for fishpond fertilization. Grab samples of the wastewater may be used to measure the faecal coliform concentration if wastewater exists.
Estimation of Water Flows and BOD Concentrations...
The mean water flows should be carefully estimated, since they have direct effects on the size of the ponds and the construction costs. A suitable design is 85 % of the in-house water consumption. The BOD may be measured if wastewater
exists, based on 24-hour flow weighted data. Alternatively, the BOD may be estimated from the following equation:
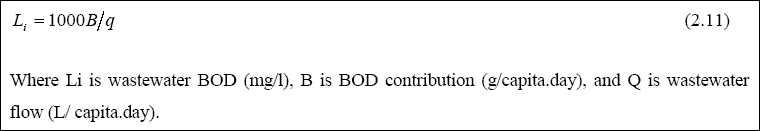
Values of B vary between 30 to 70 g/capita.day, with rich communities producing more BOD than poor communities (Campos and von Sperling, 1996). In medium-sized towns, a value of 50 g/capita.day is more suitable (Mara and Pearson, 1987). A typical design figure for an urban area in a developing country would be 40 - 50 grams BOD5 / capita . day
(Arthur, 1976). Although it is dangerous to generalize, in view of the wide variations which can be expected with differing social customs, religion, etc., a BOD5 per capita contribution of 40 grams/day, with a wastewater contribution
of about 100 liters / capita . day is probably a reasonable initial estimate where there is a household water supply; however, flows also may be considerably less. The usual range of faecal coliform in domestic wastewater is 107 – 108 faecal coliform / 100 ml, with a suitable deign value being 5 x 107 / 100 ml.
Design of Anaerobic Ponds...
The anaerobic ponds are designed on the basis of volumetric loading (LAMBDAV, g / m3. day), which is given by:
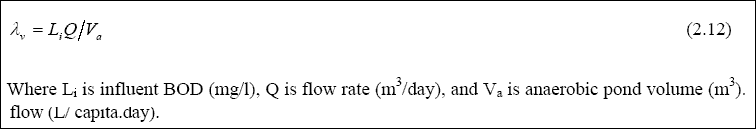
Meiring et al., 1998 recommended that the loading should be between 100 – 400 g / m3. day, in order to maintain anaerobic conditions. Once the organic loading is selected, the volume of the pond is then determined with the use of Equation 2.12. The hydraulic retention time is then calculated, using Equation 2.13, as follows:
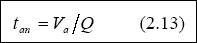
A retention time less than one day should not be used for anaerobic ponds; if it occurs, however, a retention time of one
day should be used, and the volume of the pond should be recalculated. Table 2.7 illustrates the permissible loadings to
the anaerobic ponds.
Table 2.7 Design value of permissible volumetric BOD loadings on,
and percentage BOD removal in, anaerobic ponds at various temperatures
(from Mara and Pearson, 986; Mara et al. 1997)
 Design of Facultative Ponds...
Kinetic Models for the Design of Facultative Ponds...
Facultative ponds can be designed on the basis of kinetic or empirical models. The rate at which the organic matter is oxidized by bacteria is a fundamental parameter in the rational design of biological wastewater treatment systems. It
has been found that BOD removal often approximates first-order kinetics; that is, the rate of BOD removal (rate of
oxidation of organic matter) at any time is proportional to the quantity of BOD (organic matter) present in the system at that time. This is expressed mathematically in Equation 2.14 as:
Design of Facultative Ponds...
Kinetic Models for the Design of Facultative Ponds...
Facultative ponds can be designed on the basis of kinetic or empirical models. The rate at which the organic matter is oxidized by bacteria is a fundamental parameter in the rational design of biological wastewater treatment systems. It
has been found that BOD removal often approximates first-order kinetics; that is, the rate of BOD removal (rate of
oxidation of organic matter) at any time is proportional to the quantity of BOD (organic matter) present in the system at that time. This is expressed mathematically in Equation 2.14 as:
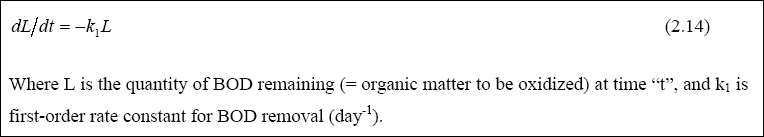
The simple approach to the rational design of facultative ponds assumes they are completely mixed reactors in which BOD5 removal follows first-order kinetics (Marais and Shaw, 1961). The rational equation for the design is illustrated in Equation 2.15:
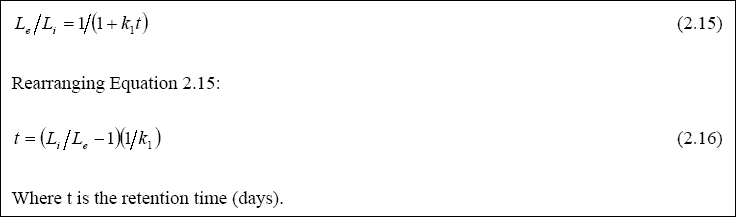

The value for k1 at 20O C was found to be 0.3 day- 1 (Mara, 1986), while the value of
kT is calculated using Equation 2.12. Note that the rate (k1) is a gross measure of bacterial
activity and, consistent with almost all parameters that describe a biological growth process, its value is strongly temperature-dependent. Its variation with temperature is usually described by an Arrhenius equation (Equation 2.19):
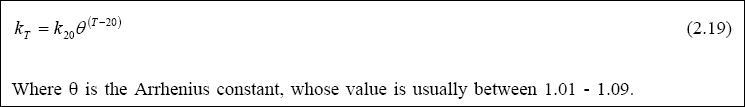
However, the typical values of THETA for the design of waste stabilization ponds range between 1.05 - 1.09. Note that the temperature should be taken as the mean temperature of the coldest month. The design example shown in the folder “Design examples” (Annex 1) illustrates how to apply the above-noted equations in designing the facultative ponds.
Empirical Models for the Design of Facultative Ponds...
Although several methods are available for designing facultative ponds, Mara (1976) recommended that facultative ponds
should be designed on the basis of surface loading (with the reasons stated in the sections above, LAMBDAS, kg / ha . day), which is give by:
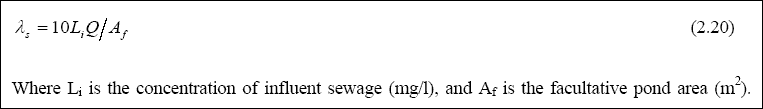
The selection of the permissible design value of LAMBDAS is usually based on the temperature. The temperature dependence indicates that the design values of LAMBDAS increases with temperature. The earliest relationship between LAMBDAS and temperature was given by McGarry and Pescode (1970), and later by Mara (1976). The Mara
(1976) equation is as shown in Equation 2.21:


A minimum retention time value of 5 days should be adopted for temperatures below 20O C, and 4 days for temperature above 20O C. This is to minimize hydraulic short-circuiting, and to give algae sufficient time to multiply (i.e., to prevent algal washout).
Design of Maturation Ponds for Faecal Coliform Removal...
The method of Marais (1974) is generally used to design a pond series for faecal coliform removal. This assumes that
faecal coliform removal can be reasonably well represented by a first-order kinetic model in a completely-mixed reactor.
The resulting equation for a single pond is given by:

Thus, kT changes by 19 % for every change in temperature of 1OC. Equation 1.20 contains the
implicit assumption that the value of kT determined from Equation 2.29 is equally valid in anaerobic,
facultative ponds. Although this is not true, Pearson et al. (1996a) nevertheless found that Equation 2.28 represents
the removal of faecal coliform in a series of ponds as a whole reasonably well. Maturation ponds require careful design
to ensure that their faecal coliform removal follows that given by Equations 2.28 and 2.29.
Examination of Equation 2.28 shows that it contains two unknowns (THETAM and n), since by this stage of
the design process, THETAA and THETAF will have been calculated, NI measured or
estimated, NE set (e.g., 1,000 / 100 ml for unrestricted irrigation), and kT calculated from
Equation 2.29. The best approach to solving Equation 2.28 is to calculate the values of THETAM
corresponding to n = 1, 2, 3, etc., and then adopt the following rules to select the most appropriate combination
of THETAM and n, namely:
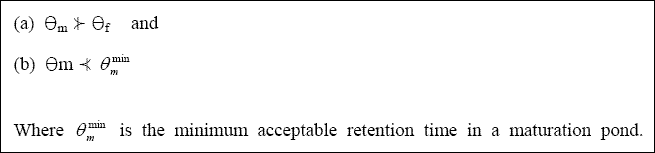
This term is introduced to minimize hydraulic short-circuiting and prevent algal washout. Marais (1974) recommends
a value of 3 days, although at temperatures below 20O C, values of 4-5 days are preferable. The remaining
pairs of THETAM and n, together with the pair THETAMMIN and n, where n is the
first value of n for which THETAM is less than THETAMMIN, are then compared, and
the one with the least product selected, since this will identify the least land area requirements. The BOD loading
on the first maturation pond must be checked, and must not be higher than that on the preceding facultative pond;
in fact, it is preferable that it be significantly lower. The maximum BOD loading in the first maturation pond
should be 75 % of that on the preceding facultative pond. It is not necessary to check the BOD loadings on subsequent maturation ponds, as the non-algal BOD contribution to the load on them is very low.
The loading on the first maturation pond is calculated on the assumption that 70 % of unfiltered BOD has been removed
in the preceding anaerobic and facultative ponds (or 80 % for temperatures above 20O C). Mara and Pearson,
(1987) also suggested that 90 % cumulative removal in anaerobic and facultative ponds, and then 25 % in each maturation
pond, for temperatures above 25O C (80 % and 20 %, respectively, for temperatures below 20O C),
when the BOD is based on filtered BOD values. Thus:

The design example in the folder “Design examples” (Annex 1) indicates how the maturation pond is designed according
to the above-noted procedures.
Helminth Egg Removal...
Helminth eggs are normally removed by sedimentation, with the process occurring in the anaerobic or primary facultative ponds. If the final effluent is to be used for restricted irrigation, it is necessary to ensure that it contains no
more than one egg per liter. Analysis of egg removal in the pond has yielded the following relation reported by Ayres
et al. (1992):

Table 2.8 Design values of percent helminth egg removal (R)
(in individual anaerobic, facultative or maturation ponds for
hydraulic retention times (THETA) in the range of 1 – 20 days,
as calculated from Equation 2.34)
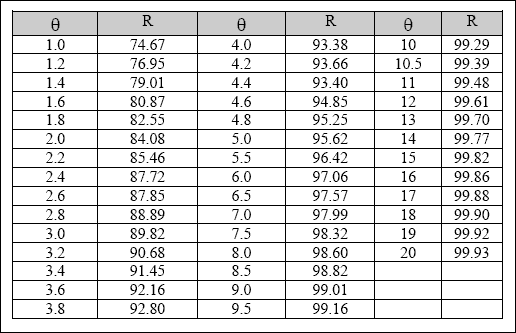 Equation 2.34 or Table 2.8 is recommended for use in the design. Annex A provides guidance on how to design a waste stabilization pond for the removal of Helminth eggs.
Design of WSP for Nutrient Removal...
The design equation for nutrient removal in WSP’s is based on equations developed in North America; thus, the designer
should use these equations with the caution that they might not accurately predict the expected performance. The equation
for ammonia-nitrogen (NH3 + NH4+) removal in individual facultative ponds was presented
by Pono and Middlebrooks (1982). The equation for temperatures below 20O C is as follows:
Equation 2.34 or Table 2.8 is recommended for use in the design. Annex A provides guidance on how to design a waste stabilization pond for the removal of Helminth eggs.
Design of WSP for Nutrient Removal...
The design equation for nutrient removal in WSP’s is based on equations developed in North America; thus, the designer
should use these equations with the caution that they might not accurately predict the expected performance. The equation
for ammonia-nitrogen (NH3 + NH4+) removal in individual facultative ponds was presented
by Pono and Middlebrooks (1982). The equation for temperatures below 20O C is as follows:
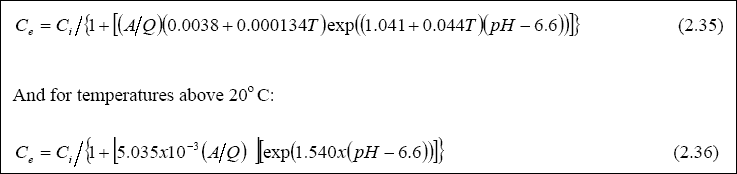
Constructed Wetlands...
General...
Constructed wetlands (CWs) are planned systems designed and constructed to employ wetland vegetation to assist in treating wastewater in a more controlled environment than occurs in natural wetlands. Hammer (1990) defines constructed wetlands as
a designed, manmade complex of saturated substrate, emergent and submerged vegetation, animal life, and water that simulate wetlands for human uses and benefits. Constructed wetlands are an “eco-friendly” alternative for secondary and tertiary municipal and industrial wastewater treatment. The pollutants removed by CW’s include organic materials, suspended solids, nutrients, pathogens, heavy metals and other toxic or hazardous pollutants. In municipal applications, they can follow traditional sewage treatment processes. Different types of constructed wetlands can effectively treat primary, secondary or tertiary treated sewage. However wetlands should not be used to treat raw sewage and, in industrial situations, the wastes may need to be pre-treated so that the biological elements of the wetlands can function effectively with the effluent. CW’s are practical alternatives to conventional treatment of domestic sewage, industrial and agricultural wastes, storm water runoff, and acid mining drainage.
Types of Constructed Wetlands...
Constructed wetlands for wastewater treatment can be categorized as either Free Water Surface (FWS) or Subsurface Flow
(SSF) systems. In FWS systems, the flow of water is above the ground, and plants are rooted in the sediment layer at the
base of water column (Figure 3.1). In SSF systems, water flows though a porous media such as gravels or aggregates, in
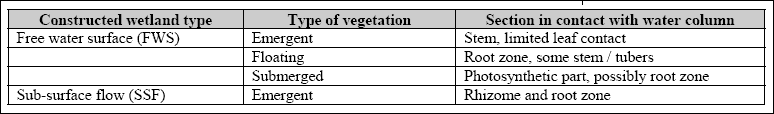
which the plants are rooted (Figure 3.2). Table 3.1 illustrates the type of wetlands, vegetation types and water column contacts in constructed wetlands.
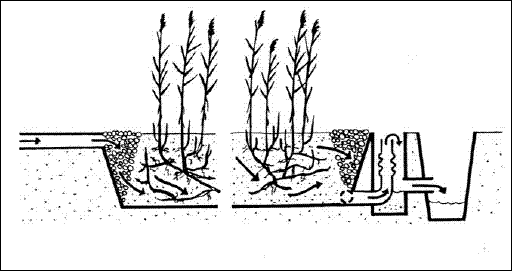
Figure 3.1 Emergent macrophyte treatment system with horizontal sub-surface flow (Brix 1993)
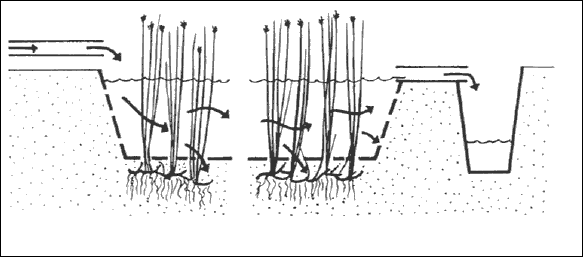
Figure 3.2 Emergent macrophytes treatment system with surface flow (Brix, 1993)
Table 3.1 Vegetation type and water column contact in constructed wetlands
 SSF systems are most appropriate for treating primary wastewater, because there is no direct contact between the water
column and the atmosphere. There is no opportunity for vermin to breed, and the system is safer from a public health perspective. The system is particularly useful for treating septic tank effluent or grey water, landfill leachate and
other wastes that require removal of high concentrations organic materials, suspended solids, nitrate, pathogens and
other pollutants. The environment within the SSF bed is mostly either anoxic or anaerobic. Oxygen is supplied by the
roots of the emergent plants and is used up in the Biofilm growing directly on the roots and rhizomes, being unlikely to penetrate very far into the water column itself. SSF systems are good for nitrate removal (denitrification), but not for ammonia oxidation (nitrification), since oxygen availability is the limiting step in nitrification. There are two types
of SSF systems: horizontal flow SSF (hSSF) and vertical flow SSF (vSSF). The most common problem with hSSF is blockage, particularly around the inlet zone, leading either to short circuiting, surface flow or both. This occurs because of poor hydraulic design, insufficient flow distribution at the inlet, and inappropriate choice of porous media for the inlet
zone. Properly-designed SSF systems are very reliable. FWS systems are very appropriate for polishing secondary and
tertiary effluents, and for providing habitat. The environment in the FWS systems is generally aerobic at, and near,
the surface, tending toward anoxic conditions near the bottom sediment. The microbial film grows on all available plant surfaces, and is the main mechanism of pollutant removal. FWS usually exhibits more biodiversity than does SSF systems.
The objective of using CWs is to remove organic matter, suspended solids, pathogenic organisms, and nutrients such as
ammonia and other forms of nitrogen and phosphorus. The growing interest in wetland system is due in part to recognition
that natural systems offer advantages over conventional activated sludge and trickling filter systems. When the same biochemical and physical processes occur in a more natural environment, instead of reactor tanks and basins, the resulting system often consumes less energy, is more reliable, requires less operation and maintenance and, as a result costs less. They also are used for removing heavy metals and toxic compounds. This manual is concerned with the design, operation and maintenance of sub-surface flow constructed wetlands.
Advantages and Disadvantages of Constructed Wetlands...
Constructed wetlands are (1) relatively inexpensive to construct and operate, (2) easy to maintain, (3) provide
effective and reliable wastewater treatment, (4) relatively tolerant of fluctuating hydrologic and contaminant loading
rates (optimal size for anticipated waste load), and (5) provide indirect benefits such as green space, wildlife habitats
and recreational and educational areas. The disadvantages are (1) the land requirements (cost and availability of suitable land), (2) current imprecise design and operation criteria, (3) biological and hydrological complexity and our lack of understanding of important process dynamics, (4) the costs of gravel or other fills, and site grading during the
construction period, and (5) possible problems with pests. Mosquitoes and other pests could be a problem for an improperly designed and managed SSF. The system may be used for small communities and, therefore, may be located close to the users.
The dependence of wetland community on hydrologic patterns is most obvious in the change in species composition resulting from alterations in water depths and flows.
Configuration, Zones and Components of Constructed Wetlands...
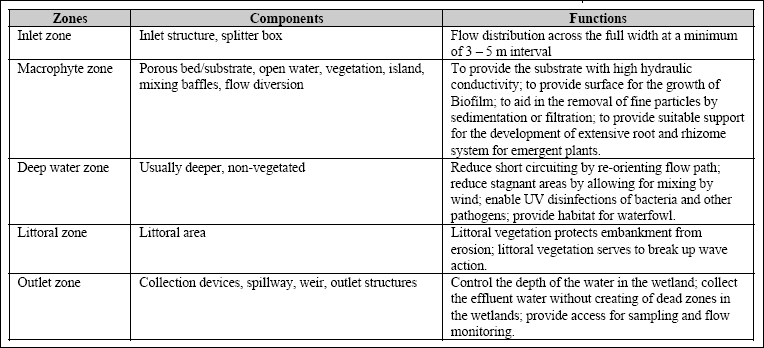
Influents to constructed wetland can range from raw wastewater to secondary effluents. Most constructed wetlands have the following zones: inlet zone, macrophyte zone, littoral zone and outlet zones. The components associated in each zones are
as shown in Table 3. 2, and can include substrates with various rates of hydraulic conductivity, plants, a water column, invertebrate and vertebrates, and an aerobic and anaerobic microbial population. The water flow is maintained approximately 15 – 30 cm below the bed surface. Plants in wastewater systems have been viewed as nutrient storage compartments where nutrient uptake is related to plant growth and production. Harvesting before senescence may permanently remove nutrients
from the systems. Within the water column, the stems and roots of wetland plants significantly provide the surface area
for the attachment of microbial population. Wetland plants have the ability to transport atmospheric oxygen and other
gases down into the root to the water column. Most media used include crushed stones, gravels, and different soils,
either alone or in combination. Most beds are underlain by impermeable materials to prevent water seepage and assure
water level control. Wastewater flows laterally, being purified during contact with media surface and vegetation roots.
The sub-surface zone is saturated and generally anaerobic, although excess DO conveyed through the plant root system
supports aerobic microsites adjacent to the root and rhizomes.
Table 3.2 Wetland zones and their associated components
SSF systems are most appropriate for treating primary wastewater, because there is no direct contact between the water
column and the atmosphere. There is no opportunity for vermin to breed, and the system is safer from a public health perspective. The system is particularly useful for treating septic tank effluent or grey water, landfill leachate and
other wastes that require removal of high concentrations organic materials, suspended solids, nitrate, pathogens and
other pollutants. The environment within the SSF bed is mostly either anoxic or anaerobic. Oxygen is supplied by the
roots of the emergent plants and is used up in the Biofilm growing directly on the roots and rhizomes, being unlikely to penetrate very far into the water column itself. SSF systems are good for nitrate removal (denitrification), but not for ammonia oxidation (nitrification), since oxygen availability is the limiting step in nitrification. There are two types
of SSF systems: horizontal flow SSF (hSSF) and vertical flow SSF (vSSF). The most common problem with hSSF is blockage, particularly around the inlet zone, leading either to short circuiting, surface flow or both. This occurs because of poor hydraulic design, insufficient flow distribution at the inlet, and inappropriate choice of porous media for the inlet
zone. Properly-designed SSF systems are very reliable. FWS systems are very appropriate for polishing secondary and
tertiary effluents, and for providing habitat. The environment in the FWS systems is generally aerobic at, and near,
the surface, tending toward anoxic conditions near the bottom sediment. The microbial film grows on all available plant surfaces, and is the main mechanism of pollutant removal. FWS usually exhibits more biodiversity than does SSF systems.
The objective of using CWs is to remove organic matter, suspended solids, pathogenic organisms, and nutrients such as
ammonia and other forms of nitrogen and phosphorus. The growing interest in wetland system is due in part to recognition
that natural systems offer advantages over conventional activated sludge and trickling filter systems. When the same biochemical and physical processes occur in a more natural environment, instead of reactor tanks and basins, the resulting system often consumes less energy, is more reliable, requires less operation and maintenance and, as a result costs less. They also are used for removing heavy metals and toxic compounds. This manual is concerned with the design, operation and maintenance of sub-surface flow constructed wetlands.
Advantages and Disadvantages of Constructed Wetlands...
Constructed wetlands are (1) relatively inexpensive to construct and operate, (2) easy to maintain, (3) provide
effective and reliable wastewater treatment, (4) relatively tolerant of fluctuating hydrologic and contaminant loading
rates (optimal size for anticipated waste load), and (5) provide indirect benefits such as green space, wildlife habitats
and recreational and educational areas. The disadvantages are (1) the land requirements (cost and availability of suitable land), (2) current imprecise design and operation criteria, (3) biological and hydrological complexity and our lack of understanding of important process dynamics, (4) the costs of gravel or other fills, and site grading during the
construction period, and (5) possible problems with pests. Mosquitoes and other pests could be a problem for an improperly designed and managed SSF. The system may be used for small communities and, therefore, may be located close to the users.
The dependence of wetland community on hydrologic patterns is most obvious in the change in species composition resulting from alterations in water depths and flows.
Configuration, Zones and Components of Constructed Wetlands...
Influents to constructed wetland can range from raw wastewater to secondary effluents. Most constructed wetlands have the following zones: inlet zone, macrophyte zone, littoral zone and outlet zones. The components associated in each zones are
as shown in Table 3. 2, and can include substrates with various rates of hydraulic conductivity, plants, a water column, invertebrate and vertebrates, and an aerobic and anaerobic microbial population. The water flow is maintained approximately 15 – 30 cm below the bed surface. Plants in wastewater systems have been viewed as nutrient storage compartments where nutrient uptake is related to plant growth and production. Harvesting before senescence may permanently remove nutrients
from the systems. Within the water column, the stems and roots of wetland plants significantly provide the surface area
for the attachment of microbial population. Wetland plants have the ability to transport atmospheric oxygen and other
gases down into the root to the water column. Most media used include crushed stones, gravels, and different soils,
either alone or in combination. Most beds are underlain by impermeable materials to prevent water seepage and assure
water level control. Wastewater flows laterally, being purified during contact with media surface and vegetation roots.
The sub-surface zone is saturated and generally anaerobic, although excess DO conveyed through the plant root system
supports aerobic microsites adjacent to the root and rhizomes.
Table 3.2 Wetland zones and their associated components
 Processes in Sub-surface Flow Constructed Wetlands (SSFCW)...
Wetland can effectively remove or convert large quantities of pollutants from point sources (municipal, industrial and agricultural wastewater) and non-point sources (mines, agriculture and urban runoff), including organic matter, suspended solids, metals and nutrients. The focus on wastewater treatment by constructed wetlands is to optimise the contact of microbial species with substrate, the final objective being the bioconversion to carbon dioxide, biomass and water.
Wetlands are characterized by a range of properties that make them attractive for managing pollutants in water (Bavor
and Adcock, 1994). These properties include high plant productivity, large adsorptive capacity of the sediments, high
rates of oxidation by microflora associated with plant biomass, and a large buffering capacity for nutrients and
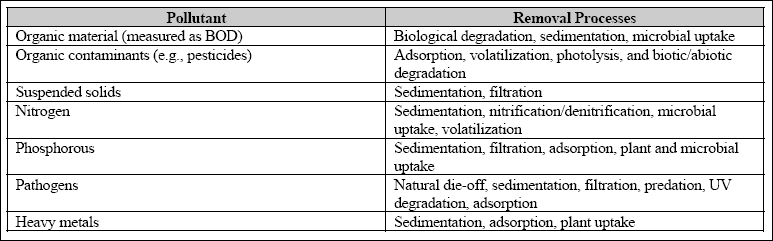
pollutants. Table 3.3 provides an overview of pollutant removal mechanisms in constructed wetlands (Mitchell, 1996).
Table 3.3 Overview of pollutant removal mechanisms
Processes in Sub-surface Flow Constructed Wetlands (SSFCW)...
Wetland can effectively remove or convert large quantities of pollutants from point sources (municipal, industrial and agricultural wastewater) and non-point sources (mines, agriculture and urban runoff), including organic matter, suspended solids, metals and nutrients. The focus on wastewater treatment by constructed wetlands is to optimise the contact of microbial species with substrate, the final objective being the bioconversion to carbon dioxide, biomass and water.
Wetlands are characterized by a range of properties that make them attractive for managing pollutants in water (Bavor
and Adcock, 1994). These properties include high plant productivity, large adsorptive capacity of the sediments, high
rates of oxidation by microflora associated with plant biomass, and a large buffering capacity for nutrients and
pollutants. Table 3.3 provides an overview of pollutant removal mechanisms in constructed wetlands (Mitchell, 1996).
Table 3.3 Overview of pollutant removal mechanisms
 Biological Processes...
There are six major biological reactions involved in the performance of constructed wetlands, including
photosynthesis, respiration, fermentation, nitrification, denitrification and microbial phosphorus removal
(Mitchell, 1996b). Photosynthesis is performed by wetland plants and algae, with the process adding carbon and
oxygen to the wetland. Both carbon and oxygen drive the nitrification process. Plants transfer oxygen to their
roots, where it passes to the root zones (rhizosphere). Respiration is the oxidation of organic carbon, and is
performed by all living organisms, leading to the formation of carbon dioxide and water. The common microorganisms
in the CW are bacteria, fungi, algae and protozoa. The maintenance of optimal conditions in the system is required
for the proper functioning of wetland organisms. Fermentation is the decomposition of organic carbon in the absence
of oxygen, producing energy-rich compounds (e.g., methane, alcohol, volatile fatty acids). This process is often
undertaken by microbial activity. Nitrogen removal by nitrification/denitrification is the process mediated by microorganisms. The physical process of volatilization also is important in nitrogen removal. Plants take up the
dissolved nutrients and other pollutants from the water, using them to produce additional plant biomass. The nutrients
and pollutants then move through the plant body to underground storage organs when the plants senesce, being deposited
in the bottom sediments through litter and peat accretion when the plants die. Wetland microorganisms, including bacteria
and fungi, remove soluble organic matter, coagulate colloidal material, stabilize organic matter, and convert organic
matter into various gases and new cell tissue (Mitchell, 1996a). Many of the microorganisms are the same as those occurring in conventional wastewater treatment systems. Different types of organisms, however, have specific tolerances and requirements for dissolved oxygen, temperature ranges and nutrients.
Chemical Processes...
Metals can precipitate from the water column as insoluble compounds. Exposure to light and atmospheric gases can break
down organic pesticides, or kill disease-producing organisms (EPA, 1995). The pH of water and soils in wetlands exerts a strong influence on the direction of many reactions and processes, including biological transformation, partitioning of ionized and un-ionised forms of acids and bases, cation exchange, solid and gases solubility.
Physical Processes...
Sedimentation and filtration are the main physical processes leading to the removal of wastewater pollutants. The effectiveness of all processes (biological, chemical, physical) varies with the water residence time (i.e., the length of time the water stays in the wetland). Longer retention times accelerate the remove of more contaminants, although too-long retention times can have detrimental effects.
Limitations of Wetland Processes...
Process rates : The chemical and biological processes occur at a rate dependent on environmental factors,
including temperature, oxygen and pH. Metabolic activities are decreased by low temperature, reducing the effectiveness
of pollutant uptake processes relying on biological activity. Low oxygen concentrations limit the processes involving
aerobic respiration within the water column, and may enhance anaerobic processes, which can cause further degradation
of water quality. Many metabolic activities are pH-dependent, being less effective if the pH is too high or low.
Biological Processes...
There are six major biological reactions involved in the performance of constructed wetlands, including
photosynthesis, respiration, fermentation, nitrification, denitrification and microbial phosphorus removal
(Mitchell, 1996b). Photosynthesis is performed by wetland plants and algae, with the process adding carbon and
oxygen to the wetland. Both carbon and oxygen drive the nitrification process. Plants transfer oxygen to their
roots, where it passes to the root zones (rhizosphere). Respiration is the oxidation of organic carbon, and is
performed by all living organisms, leading to the formation of carbon dioxide and water. The common microorganisms
in the CW are bacteria, fungi, algae and protozoa. The maintenance of optimal conditions in the system is required
for the proper functioning of wetland organisms. Fermentation is the decomposition of organic carbon in the absence
of oxygen, producing energy-rich compounds (e.g., methane, alcohol, volatile fatty acids). This process is often
undertaken by microbial activity. Nitrogen removal by nitrification/denitrification is the process mediated by microorganisms. The physical process of volatilization also is important in nitrogen removal. Plants take up the
dissolved nutrients and other pollutants from the water, using them to produce additional plant biomass. The nutrients
and pollutants then move through the plant body to underground storage organs when the plants senesce, being deposited
in the bottom sediments through litter and peat accretion when the plants die. Wetland microorganisms, including bacteria
and fungi, remove soluble organic matter, coagulate colloidal material, stabilize organic matter, and convert organic
matter into various gases and new cell tissue (Mitchell, 1996a). Many of the microorganisms are the same as those occurring in conventional wastewater treatment systems. Different types of organisms, however, have specific tolerances and requirements for dissolved oxygen, temperature ranges and nutrients.
Chemical Processes...
Metals can precipitate from the water column as insoluble compounds. Exposure to light and atmospheric gases can break
down organic pesticides, or kill disease-producing organisms (EPA, 1995). The pH of water and soils in wetlands exerts a strong influence on the direction of many reactions and processes, including biological transformation, partitioning of ionized and un-ionised forms of acids and bases, cation exchange, solid and gases solubility.
Physical Processes...
Sedimentation and filtration are the main physical processes leading to the removal of wastewater pollutants. The effectiveness of all processes (biological, chemical, physical) varies with the water residence time (i.e., the length of time the water stays in the wetland). Longer retention times accelerate the remove of more contaminants, although too-long retention times can have detrimental effects.
Limitations of Wetland Processes...
Process rates : The chemical and biological processes occur at a rate dependent on environmental factors,
including temperature, oxygen and pH. Metabolic activities are decreased by low temperature, reducing the effectiveness
of pollutant uptake processes relying on biological activity. Low oxygen concentrations limit the processes involving
aerobic respiration within the water column, and may enhance anaerobic processes, which can cause further degradation
of water quality. Many metabolic activities are pH-dependent, being less effective if the pH is too high or low.
Hydrological limitations : The capacity of wetlands to treat wastewater is limited, both in terms of the quantity
of water, and the total quantity of the pollutants. Hydraulic overloading occurs when the water flow exceeds the design capacity, causing a reduction in water retention time that affects the rate of pollutant removal. Pollutant overloading occurs when the pollutant input exceeds the process removal rates within the wetland (White et al., 1996). Hydraulic overloading may be compensated for by using surcharge mechanisms, or the design may be based on a flush principle,
whereby large water flows bypass the wetland when used for storm water treatment (White et al., 1996). Inflow variations
are typically less extreme for wetlands treating municipal wastewaters, with incoming pollutant loads also being more
defined and uniform.
Wetland Nitrogen Processes...
The most important nitrogen species in wetlands are dissolved ammonia (NH4+), nitrite (NO2-), and nitrate (NO3-). Other forms include nitrous oxide gas (N2O), nitrogen gas (N2), urea (organic), amino acids and amine (Kadlec & Knight, 1996).
Total nitrogen in any system is referred to as the sum of organic nitrogen, ammonia, nitrate and nitrous gas (Organic-N + NH4+ + NO3- + N2O). The various nitrogen forms are continually involved in transformations from inorganic to organic compounds, and vice-versa. Many of these transformations are biotic, being carried out by nitrobacter and nitrosomonas (Kadlec & Knight, 1996). As it undergoes its various transformations, nitrogen is taken up by wetland plants and microflora (preferentially as NH4+, and NO3-), some is leached to the subsoil, some is liberated as gas to the atmosphere, and some
flows out of the wetland, normally in a dissolved form. Organic nitrogen comprises a significant fraction of wetland
biota, detritus, soils, sediments and dissolved solids (Kadlec and Knight, 1996). It is not readily assimilated by aquatic plants, and must be converted to NH4+, or NO3- through multiple conversions requiring long reaction time (Kadlec & Knight, 1996). The process of biological nitrogen removal follows several sequences. Nitrification first takes place, generally in the rhizosphere and in biofilms (aerobic process). Denitrification may then follow, occurring in soils and below the oxidized microzone at the soil/water interface, as it is an anaerobic process (Broderick et al., 1989). Nitrification is a two-step process catalysed by Nitrosomonas and nitrobacter bacteria. In
the first step, ammonia is oxidized to nitrite in an aerobic reaction catalyzed by Nitrosomonas bacteria, as shown in Equation 3.1:

The first reaction produces hydroxonium ions (acid pH), which react with natural carbonate to decrease the alkalinity (Mitchell, 1996a). In order to perform nitrification, the nitrosomonas must compete with heterotrophic bacteria for
oxygen. The BOD of the water must be less than 20 mg/l before significant nitrification can occur (Reed et al., 1995). Temperatures and water retention times also may affect the rate of nitrification in the wetland. Denitrification is the process in which nitrate is reduced in anaerobic conditions by the benthos to a gaseous form. The reaction is catalyzed
by the denitrifying bacteria Pseudomonas spp. and other bacteria, as follows:
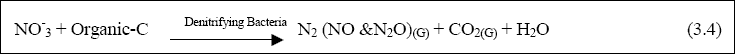
Denitrification requires nitrate, anoxic conditions and carbon sources (readily biodegradable). Nitrification must
precede denitrification, since nitrate is one of the prerequisites. The process of denitrification is slower under
acidic condition. At a pH between 5-6, N20 is produced. For a pH below 5, N2 is the main
nitrogenous product (Nuttall et al., 1995). NH4+ is the dominant form of ammonia-nitrogen at
a pH of 7, while NH3 (present as a dissolved gas) predominates at a pH of 12. Nitrogen cycling within,
and removal from, the wetlands generally involves both the translocation and transformation of nitrogen in the wetlands, including sedimentation (resuspension), diffusion of the dissolved form, litter fall, adsorption/desorption of soluble nitrogen to soil particles, organism migration, assimilation by wetland biota, seed release, ammonification (mineralisation) (Orga-N – NH4+), ammonia volatilization (NH4+ – NH3 (gas), bacterially-mediated nitrification/denitrification reactions, nitrogen fixation (N2, N2O (gases – organic-N), and nitrogen assimilation by wetland biota (NH4+, NOx organic – N, with NOx usually as NO3-). Precipitation is not a significant process due to the high solubility of nitrogen, even in inorganic form. Organic nitrogen comprises a significant fraction of wetland biota, detritus, soils, sediments and
dissolved solids (Kadlec and Knight, 1996).
Phosphorus Removal...
Phosphorus is an essential requirement for biological growth. An excess of phosphorus can have secondary effects by triggering eutrophication within a wetland, and leading to algal blooms and other water quality problems. Phosphorus may enter a wetland in dissolved and particulate forms. It exits wetlands in outflows, by leaching into the sub-soil, and by removal by plant and animals. Phosphorus removal in wetlands is based on the phosphorous cycle, and can involve a number
of processes. Primary phosphorus removal mechanisms include adsorption, filtration and sedimentation. Other processes
include complexation/precipitation and assimilation/uptake. Particulate phosphorus is removed by sedimentation, along
with suspended solids. The configuration of constructed wetlands should provide extensive uptake by Biofilm and plant
growth, as well as by sedimentation and filtration of suspended materials. Phosphorus is stored in the sediments, biota, (plants, Biofilm and fauna), detritus and in the water. The interactions between compartments depend on environmental conditions such as redox chemistry, pH and temperature. The redox status of the sediments (related to oxygen content) and litter/peat compartment is a major factor in determining which phosphorus cycling processes will occur. Under low oxygen conditions (low redox potential), phosphorus is liberated from the sediments and soils back into the water column, and can leave the wetland if the anaerobic condition is not reversed (Moss et al., 1986).
Suspended Solids...
Solids may be derived from outside a wetland (e.g., inflows and atmospheric inputs), and from within a wetland from
plankton (zooplankton and phytoplankton), and plant and animal detritus. With low wetland water velocities and
appropriate composition of influent solids, suspended solids will settle from the water column within the wetland.
Sediment resuspension not only releases pollutants from the sediments, it increases the turbidity and reduces light penetration. The physical processes responsible for removing suspended solids include sedimentation, filtration,
adsorption onto Biofilm and flocculation/precipitation. Wetland plants increase the area of substrate available for development of the Biofilm. The surface area of the plant stems also traps fine materials within its rough structure.
Pathogen Removal...
Pathogens are disease-causing organisms (e.g., bacteria, viruses, fungi, protozoa, helminthes). Wetlands are very
effective at removing pathogens, typically reducing pathogen number by up to five orders of magnitude from wetland
inflows (Reed at al., 1995). The processes that may remove pathogens in wetlands include natural die-off, sedimentation, filtration, ultra-violet light ionization, unfavorable water chemistry, temperature effects, predation by other organisms
and pH (Kadlec & knight 1996). Kadlec and Knight (1996) showed that vegetated wetlands seem more effective in pathogen removal, since they allow a variety of microorganisms to grow which may be predators to pathogens.
Heavy Metal Removal...
Heavy metals is a collective name given to all metals above calcium in the Periodic Table of Elements, which can be
highly toxic, and which have densities greater that 5g/cm3 (Skidmore and Firth, 1983). The main heavy metals of concern
in freshwater include lead, copper, zinc, chromium, mercury, cadmium and arsenic. There are three main wetland processes
that remove heavy metals; namely, binding to soils, sedimentation and particulate matter, precipitation as insoluble salts, and uptake by bacteria, algae and plants (Kadlec & Knight, 1996). These processes are very effective, with removal rates reported up to 99 % (Reed et al., 1995). A range of heavy metals, pathogens, inorganic and organic compounds present in wetlands can be toxic to biota. The response of biota depends on the toxin concentration and the tolerance of organisms
to a particular toxin. Wetlands have a buffering capacity for toxins, and various processes dilute and break down the
toxins to some degree.
Abiotic Factors and their Influence on Wetlands...
Oxygen...
Oxygen in wetland systems is important for heterotrophic bacterial oxidation and growth. It is an essential component for many wetland pollutant removal processes, especially nitrification, decomposition of organic matter, and other biological mediated processes. It enters wetlands via water inflows or by diffusion on the water surface when the surface is turbulent. Oxygen also is produced photosynthetically by algae. Plants also release oxygen into the water by root exudation into the root zone of the sediments. Many emergent plants have hollow stems to allow for the passage of oxygen to their root tissues. The oxygen-demand processes in wetlands include sediment-litter oxygen demand (decomposition of detritus), respiration (plants/animals), dissolved carbonaceous BOD, and dissolved nitrogen that utilizes oxygen through nitrification processes (Kadlec & Knight, 1996). The oxygen concentration decreases with depth and distance from the water inflow into the wetland. It is typically high at the surface, grading to very low in the sediment –water interface.
pH...
The pH of wetlands is correlated with the calcium content of water (pH 7 = 20 mg Ca/L). Wetland waters usually have a pH of around 6-8 (Kadlec and Knight, 1996). The biota of wetlands especially can be impaired by sudden changes in pH.
Temperature...
Temperature is a widely-fluctuating abiotic factor that can vary both diurnally and seasonally. Temperature exerts a strong influence on the rate of chemical and biological processes in wetlands, including BOD decomposition, nitrification and denitrification.
Process Design for Constructed Wetlands...
Hydrology...
It is not safe to ignore water exchanges with the atmosphere, mainly because they can significantly contribute to water flows. Rain causes two opposing effects, including (1) dilution of waters, thereby reducing material concentrations, and
(2) increased water velocity, increasing the water retention time within a wetland. The presence of vegetation may retard
the evapotranspiration, although wetland evapotranspiration is usually 0.8 times the Class A pan set at an adjacent open site. Preparation of an accurate hydrological budget is needed to properly design a constructed wetland. The water balance
to a wetland can be calculated as follows:

Design Approaches for Constructed Wetlands...
The characteristic wastewater parameters to be treated by the CW include BOD, COD, suspended solids (SS), nitrogen
compounds, phosphorus compounds, heavy metals, and pathogenic organisms. Three approaches have been used to design constructed wetlands. An empirical approach is based on two different “rule of thumb” approaches (Reed et al., 1995;
Kadlec and Knight 1996). In the United Kingdom, constructed wetlands have been designed using a rule of thumb method.
In contrast, both Reed et al. (1995) and Kadlec & Knight (1996) consider wetlands as attached-growth biological reactors, therefore using a first-order plug flow kinetics model as the basis for their performance equations. The removal of
soluble BOD in the SSFCW is due to microbial growth attached to the plant roots, stems, leaf litter and substrates.
Both Reed et al. (1995) and Kadlec and Knight (1996) admit that BOD5 removal in SSFCW can be described with first-order plug flow kinetics. First-order kinetics simply means that the rate of removal of a particular pollutant is direct proportional to the remaining concentration at any point within the wetland cell. Two idealized mixing theories
may be applied:
• Completely mixed reactor : The concentration is the same as the effluent concentration at any point in the reactor;
• Plug flow : The concentration of the reactant decreases along the length of the flow path through the reactor.
Plug flow obviously provides a more appropriate description of the flow pattern in constructed wetlands.
The main difference between Reed’s approach and that of Kadlec and Knight is the basis for their choice of rate constants. Reed’s equations use a volumetric, temperature-dependent basis, with the calculations being based on the available volume
of the wetland and the average water temperature. Kadlec and Knight’s equations assume an areal basis; thus, the rate constant is related only to the wetland surface area, and temperature changes are considered significant only for nitrogen removal. One of the limitations of the Kadlec and Knight approach is that, as the required effluent concentration approaches the minimum pollutant concentration, their predicted area for the treatment increases exponentially, leading to severe over-estimates of the required area in cases near the pollutant concentration limit.
Kadlec and Knight’s rate constants are the result of analyzing the performance of all the FWS and SSF constructed wetlands listed in the North American Data Base (NADB), which were not designed using the first-order plug flow approximation. It should be noted that Kadlec and Knight’s statistical analysis of the available data is comprehensive, and the authors point out that caution should be used in applying their so-called “global” parameter values to new systems. Reed included temperature-dependence term for all pollutant of general interest in the design of constructed wetland.
Reed’s Method for the Design of Constructed Wetlands...
The equations of Reed et al. (1995) are based on the first-order plug flow assumption for those pollutants that are
removed primarily by biological processes, including biochemical oxygen demand (BOD), ammonia (NH4+) and nitrate (NO3-). Reed suggests separate equations for total suspended solids (TSS) and total phosphorus (TP), based on regression analyses of an early version of the NADB (Knight et al., 1993) for constructed
wetlands. For the removal of pathogenic organisms in constructed wetlands, he suggests the same approach as that used for waste stabilization ponds. The design equations based on Reed et al. (1995) are as presented below:
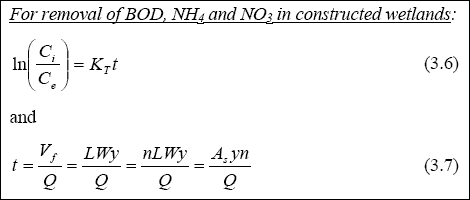
The actual retention time in constructed wetlands is a function of the porosity of the substrate used, as defined by the third and fourth expression in Equation 3.7. The substrate porosity may be obtained as n = Vv / V. The value n
is defined as the remaining cross-sectional area available for flow:
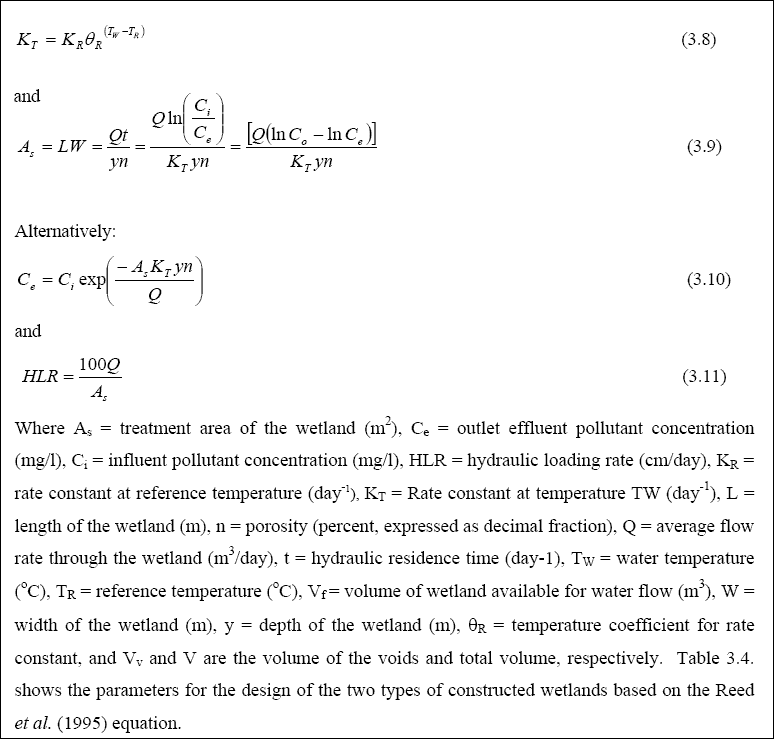
Table 3.4 Temperature coefficient for rate constant for Reed et al. (1995a) design equations
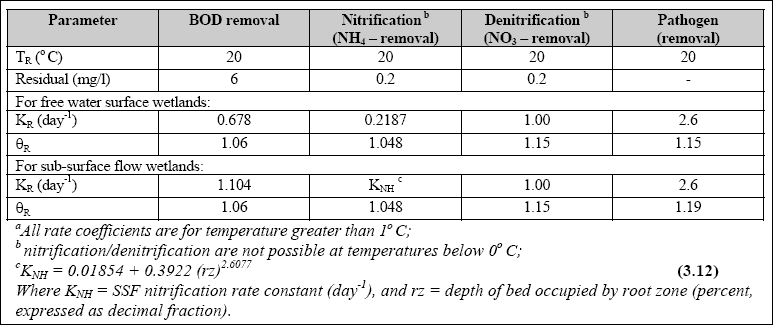

For Pathogen Removal : Reed argues that the mechanisms for pathogen removal are essentially the same in both
waste stabilization ponds and constructed wetlands. Where pathogen removal has been investigated in constructed wetlands, Equation 10 has proven to be conservative; hence, it is useful as a predictor:
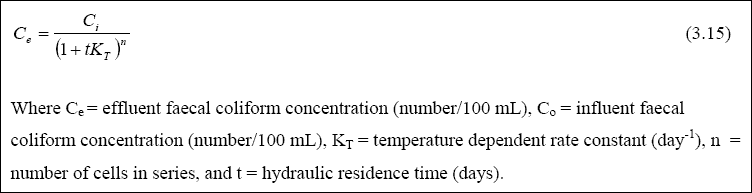
The reliance of this formula on the number of cells in series tends to suggest that, for optimal pathogen removal, the
number of cells should be maximised.
For TP removal : In both SSF and FWS wetlands :


Kadlec and Knight Design Method...
Kadlec and Knight (1996) presume a first-order decay, plug flow model for all pollutants, including BOD, TSS, total phosphorous (TP), total nitrogen (TN), organic nitrogen (Org - N), ammonia nitrogen (NH4+ - N), oxidised nitrogen (NOX - N), and faecal coliform (FC). Their model is based on areal rate constants, which
are independent of temperature. The Kadlec and Knight model may be less sensitive to different climatic conditions:
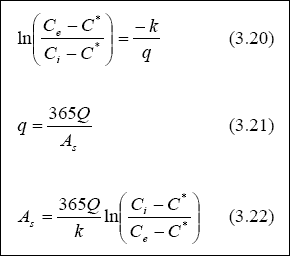
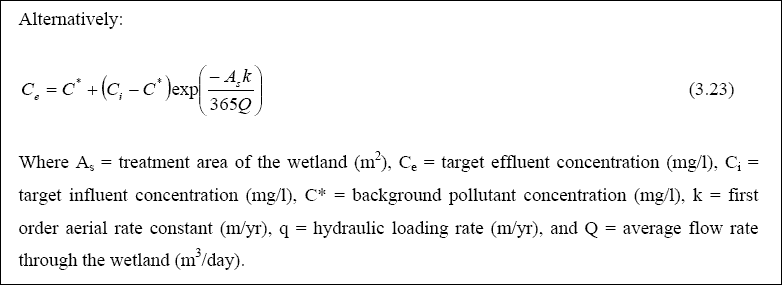
Kadlec and Knight (1996) advocate the use of the “global” parameters they determined from plug flow analysis of performance data available to date on the North American Data Base (NADB) (Knight et al., 1993) in other systems. They suggest that specific parameters should be locally determined prior to investment in a full-scale system, in order to ensure suitability of design. The global parameters to be used for this design are shown in Table 3.5. Where values for q other than 1.00 are given, it indicates that the parameter value varies with temperature. Systems layout : Components of the CW include preliminary/primary treatment units and the CWs cell (or cells). The CW includes substrate, vegetation and biological organisms contained within the physical configuration that can be described as an attached growth biological filter. Configuration : Configuration should enhance wastewater distribution to maximize contact between wastewater,
substrate and vegetation by minimizing short-circuiting. The configuration should consider the degree of pre-treatment, required treatment area, available land shape and slope, length- to-width ratio, desired bed slope, amount of excavation
and grading to obtain cell depth and slope, substrate type, collection pipes, and operation and maintenance flexibility. There are three possible configurations for wetland cells used for wastewater treatment: In parallel, in series, or a combination of the two. The main advantage for cells in parallel is the flexibility and redundancy in operation, so that individual cells can easily be taken off-line for maintenance and repair. The water flow can be re-directed to other cells, allowing for ongoing operation of the wastewater treatment plant and the wetland. When the wetland cells operate in series, the water moves sequentially from one cell to next, forming a chain of wetland cells. The main operational advantage of
cells in series is minimization of short-circuiting, leading to better overall treatment in the system. From the reactor theory, an ideal plug provides the best pollutant removal. The selected configuration must be based on a clear understanding of the objectives, influent water quality, desired effluent water quality, hydraulic regime and site specific constraints
and opportunities (Kadlec and Knight, 1996). An L/W value as low as 1 is recommended for SSF (Hammer, 1990), while an L/W ratio of at least 10 is required for the SF. The bed slope for SSF should be 2 % or less, while that for SF should be 0.5 %
or less. The substrate depth should be 0.6 m. Preliminary / primary treatment : CWs should be preceded with screens, or comminution as preliminary treatment facilities. Imhoff tanks, septic tanks and stabilization pond(s) should be used as primary treatment facilities. Small communities with water flow rates less than 380 m3 / day may use septic
tanks as primary treatment facility to reduce organic solids.
Table 3.5 Preliminary model parameter values for Kadlec and Knight’s design equations (Kadlec and Knight, 1996)
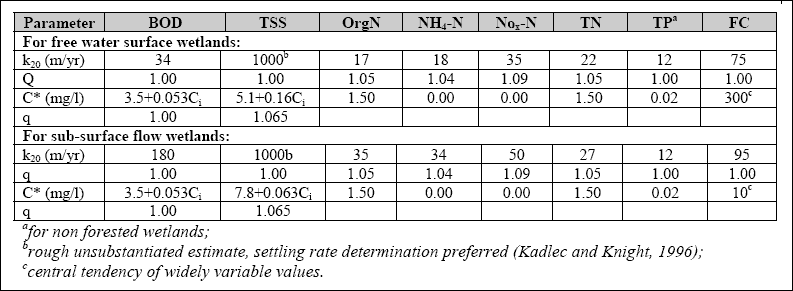 Operation and Maintenance of Waste Stabilization Ponds...
The operation of the ponds includes the commissioning (starting up), maintaining and monitoring of its performance.
Start-up Phase for Waste Stabilization Ponds...
Anaerobic ponds must be filled with raw sewage and seeded with sludge from conventional sewage treatment plants or septic tanks. After filling and seeding, the pond should gradually be loaded up to the design-loading rate. The pond contents
should have a pH above 7, to allow the development of methanogenic bacteria. Lime or soda ash is added, if necessary, to
rise the pH in the pond. If the sewerage system is new, and the flow rate, as well as the loading rate, to the anaerobic
pond is low, the sewage may be bypassed till the flow rate and the loading rate from the sewerage systems satisfies the condition to be discharged in the pond. It is important to have a bypass from the anaerobic pond that will be used during de-sludging. It is also recommended that the ponds be commissioned during the beginning of the hot season, in order to
allow the quick establishment of microorganisms of importance for the waste stabilization ponds. The facultative pond
should be commissioned before the anaerobic pond, in order to avoid odors during the release of anaerobic pond effluent
to an empty facultative pond. During the start-up phase of the facultative and maturation ponds, the ponds should be filled with freshwater (from tap, river or wells), thereby allowing the gradual development of algae and heterotrophic bacteria population. However, the primary facultative pond must be seeded as an anaerobic pond. If freshwater is not available,
the secondary facultative and maturation ponds should be filled with raw sewage and left for 3-4 weeks, to allow the development of the microbial population.
Maintenance...
Maintenance of the ponds should be carried out regularly to avoid odors, flies and mosquito nuisances. The routine maintenance includes :
Operation and Maintenance of Waste Stabilization Ponds...
The operation of the ponds includes the commissioning (starting up), maintaining and monitoring of its performance.
Start-up Phase for Waste Stabilization Ponds...
Anaerobic ponds must be filled with raw sewage and seeded with sludge from conventional sewage treatment plants or septic tanks. After filling and seeding, the pond should gradually be loaded up to the design-loading rate. The pond contents
should have a pH above 7, to allow the development of methanogenic bacteria. Lime or soda ash is added, if necessary, to
rise the pH in the pond. If the sewerage system is new, and the flow rate, as well as the loading rate, to the anaerobic
pond is low, the sewage may be bypassed till the flow rate and the loading rate from the sewerage systems satisfies the condition to be discharged in the pond. It is important to have a bypass from the anaerobic pond that will be used during de-sludging. It is also recommended that the ponds be commissioned during the beginning of the hot season, in order to
allow the quick establishment of microorganisms of importance for the waste stabilization ponds. The facultative pond
should be commissioned before the anaerobic pond, in order to avoid odors during the release of anaerobic pond effluent
to an empty facultative pond. During the start-up phase of the facultative and maturation ponds, the ponds should be filled with freshwater (from tap, river or wells), thereby allowing the gradual development of algae and heterotrophic bacteria population. However, the primary facultative pond must be seeded as an anaerobic pond. If freshwater is not available,
the secondary facultative and maturation ponds should be filled with raw sewage and left for 3-4 weeks, to allow the development of the microbial population.
Maintenance...
Maintenance of the ponds should be carried out regularly to avoid odors, flies and mosquito nuisances. The routine maintenance includes :
• Removing screenings and grit from the inlet and outlet works;
• Cutting grasses on the embankment, and removing it so that it does not fall in the ponds;
• Removing floating scum and floating macrophytes
from the surface of the maturation and facultative ponds;
• Spraying scum on the surface of the anaerobic ponds and
not removing it, since this will help the treatment processes;
• Removing any accumulated solids in the inlet and
outlet works;
• Repairing any damaged embankment as soon as possible; and
• Repairing any damage of the fences or gates.
The operator must be given precise information on what must be done at the pond site. A clear form should be prepared, clearly showing the tasks to be performed by the operator, and which should be counter-checked weekly by the foreman/supervisor. Mara (1987) recommends that, for proper pond operation, there should be a manager, assistant manager, engineers, work foreman, laboratory chemist, assistant laboratory chemist, technicians, artisans and clerks. The number of staff members will depend on how extensive the project is, as well as the population to be served. Anaerobic ponds require desludging when they are one third full of the sludge by volume.
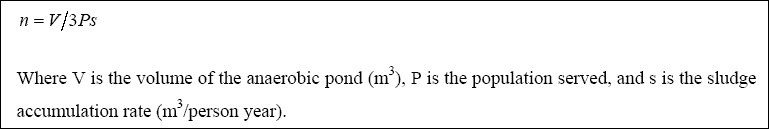
The value of “s” is usually 0.04 m3 / person . year. The precise time to de-sludge the anaerobic pond is
sometimes determined by the use of a “white towel.” Mara (1987) recommends an annual de-sludging frequency. The sludge
from the pond may be disposed of in sludge lagoons or tankers that transport it to a landfill site, agricultural land or other suitable disposal area. The sludge disposal should be done in accordance with local regulations.
Monitoring and Evaluation of Performance...
Frequent monitoring of the final effluent quality of a pond system is required to address the following needs :
• Regular assessment regarding whether or not the effluent complies with the local discharge or reuse standards;
and
• Detection of any sudden failure, or determining if the pond effluent has started to deteriorate; it also may
help identify the causes of the problem(s) and the remedial actions to be taken. Two levels of monitoring are recommended. Level 1 involves taking samples of the final effluent at least monthly or weekly, for analysis of the parameters for which effluent discharge or reuse requirements exist. Level 2 is implemented when the Level 1 monitoring indicates that the effluent is failing to meet necessary standards, thereby requiring a more detailed study. Twenty-four hour flow-weighted composite samples are preferable for most of the parameters to be analyzed, although grab samples are necessary for some of them (pH, temperature, faecal coliform). The methods for collecting composite samples are as follows :
• Automatic sampler : which takes samples every 1-2 hours, with subsequent manual flow weighting if this is not done automatically;
• Grab samples : every 1-3 hours, with subsequent manual flow weighting; and
• Column samples : near the final
pond outlet. A full evaluation of the performance of the pond is a time-consuming, expensive process, requiring experienced personnel to interpret the results. Details on how to collect samples for evaluating the performance of the pond is given
by Mara (1987). Evaluation of the pond performance has the following significance :
• Provides information on how the pond is either under-loaded or overloaded;
• Indicates how much loading may safely be added to the system as the community increases; and
• Indicates how the future design can be improved in the region.
Operation and Maintenance of Constructed Wetlands...
Commissioning...
Sometimes commissioning of the wetland is referred as the time from planting to the date where the wetland is considered operational. Operation during this period should ensure an adequate cover of the wetland vegetation. The water level within the wetland during this time needs to be controlled carefully, to prevent seedling from being desiccated or drowned. Once
the plants are established, the water level may be raised to operational level. Plant loss may occur during the commissioning, therefore requiring transplanting.
Operation...
The operation of a constructed wetland depends on the type of wetland, and the number of preliminary treatment units used
for wastewater treatment. Constructed wetlands are designed to be passive and low maintenance, thereby not requiring continual upkeep. Constructed wetland, however, are dynamic ecosystems, with many variables that require managing. If not, problems may occur when the operator does not understand the needed operation and maintenance, the wetland is either hydraulically or organically overloaded, unavoidable disasters (e.g., flooding, drought) occur, the wetland is plagued by weed problems and/or if excessive quantities of sediments, litter and pollutants accumulate and are not removed from the wetland. The management of the constructed wetlands consists of four tasks, as outlined below in Table 4.1 Not all constructed wetlands are the same, given that they can be designed for a range of objectives. These objectives will
determine the kind of operation and management activities needed to be undertaken. Thus, the operation and management of a constructed wetland must be tailored to a particular constructed wetland, reflecting desired objectives and site-specific constraints, including local hydrology, salinity, climate and relevant aspects of public safety. The essential elements
of the operation and maintenance of a constructed wetland must include :
• Description of the wetland and its
objectives;
• List of tasks or management; and
• Monitoring activities, including inspection checklists.
Table 4.1 Management of constructed wetlands
 The operation of a constructed wetland after commissioning must include :
The operation of a constructed wetland after commissioning must include :
• Maintaining the embankments;
• Removing litter and debris;
• Checking the water flow rate to a constructed wetland to determine if it is in accordance with the design;
• Removing any blockages in the inlet and outlet works;
• Replacing plants as required;
• Removing any unwanted weed species from the constructed wetland;
• Checking the plants for any sign of diseases;
• Protecting the deep open water;
• Correcting erosion and slumping; and
• Checking for any signs of over-flooding (for sub-surface flow constructed wetlands). These tasks may be addressed in the form of a checklist to direct the required maintenance, and to identify who should be immediately contacted in the event of problems.
Monitoring...
Monitoring selected performance parameters should provide sufficient information to measure performance in meeting wetland objectives. If water quality improvement is the primary objective, the performance indicator should either be presented as
a concentration or load at the outlet, or a comparison of inflow and outflows, also in terms of concentrations or loads. If monitoring results indicate that the system is not working according to the objectives, corrective measures must be applied. Improvement of water quality may be assessed by monitoring a range of inflow and outflow water-quality parameters. Useful parameters for monitoring wetland performance include dissolved oxygen (DO), BOD, COD, total phosphorus, orthophosphorus,
total nitrogen, total Kjeldhal nitrogen, ammonia nitrogen, oxidized nitrogen, faecal coliform, pH, suspended solids, electrical conductivity, and heavy metal concentrations. Water flow rates to and from the constructed wetland also must be measured. The sampling may be done using either an automatic or manual sampler. Samples within the wetland must sometimes
be taken for the purpose of comparison.
Decommissioning and Refitting...
Decommissioning and refitting of a constructed wetland may take place if its design lifetime is over. At the end of its design life, a wetland will be either be refitted, or decommissioned if no longer required. Refitting may be required when the accumulation of wetland sediments is adversely affecting wetland performance, or when changing catchment conditions require modifications of the wetland. Major refits may include the removal of accumulated peat, including aquatic plants, and replacements of substrates. Decommissioning of a wetland may be required if the land supporting it is utilized for other purposes, or if the wetland functioning is unable to achieve the original design objectives.
Biogeochemical Dynamic Models for Waste Stabilization Ponds and Constructed Wetlands...
Background...
A model is an idealization of a real situation, in which the most important components are identified and their
interactions described and used as a tool to solve problems (Jorgensen, 1986; Daigger, 1982). An ecological model
is regarded as a mathematical presentation of the biochemical activities relevant to the ecology of a secondary
facultative pond. Energy flows in ecosystems are uni-directional and non-cyclic, whereas nutrient movement is cyclic.
An ecosystem is a functional unit, in that its components are connected by flows of energy and matter. The type of
ecological model to be formulated is determined by the nature of the problem to be solved, the ecosystem being addressed,
and the data available (Jorgensen, 1992). In its mathematical formulation, an ecological model consists of 5 components, including: (i) Forcing functions or external variables; (ii) State variables; (iii) Mathematical equations; (iv) Parameter coefficients; and (v) Universal constants. The level of organisation of the model depends on the nature of the problem to
be solved. There are many classes of ecological models, including stochastic, deterministic, reductionist, holistic, steady-state, static, distributed, and lumped-parameter models. The level of organisation of the ecosystem, and the
external factors, help decide what type of model is suitable for describing a specific ecological system. A combination of approaches, such as empirical studies, comparative studies, and experimental modelling, should be used to provide a whole picture of an ecosystem. Ecosystems are typically so complex that it is sometimes too difficult to try to represent all the system's features accurately using only one approach. Understanding the processes and reactions of an ecosystem is a basis for evaluating the ability of nature to self-design its forcing functions, thereby providing a guide for choosing the appropriate biotechnological method. It is not only important to know how humans can influence the ecosystem processes,
and how the ecosystem components are linked, but also how changes in one ecosystem can produce changes in a neighboring ecosystem. An example in wastewater treatment is that it often is necessary to use primary treatment units, followed by secondary or biological treatment processes. Any disturbances that may occur in the primary treatment units also will
affect the secondary biological treatment processes. Waste stabilization ponds and constructed wetlands are ecosystems
with defined physical, chemical and biological processes. They are considered an ecosystem because of the interactions between their organisms and their surrounding environment. 5.1.1 Elements of ecological modelling During any formulation
of physical, biological of chemical processes of an ecosystem, model, it is necessary to know the elements of each
ecosystem, in order to have a clear understanding of what should be included in the model.
State Variables...
State variables refer to the variables that describe the state of an ecosystem, being those variables that constitute its main properties. It is the change in state variables that characterize the dynamic system. There are four ways in which a state variable can change over time (Hardisty et al., 1994): Damped -- the state variables stay at or fluctuate around a fixed level; Explosive condition -- the state variables move progressively away from an initial value; Periodic condition – the state variables move between extremes through time; unsystematic condition -- a state variable has no perceptible
pattern of changes over time (i.e., the change is chaotic).
Forcing Functions...
In this case, the forcing functions refer to variables of an external nature that influence the state variables of an ecosystem. The ecological model is used to predict what will change in an ecosystem when the forcing functions are varied with time. The forcing functions to the system that may be influenced by humans are the control functions, such as the
sewage flow rate to the pond, which may be controlled to a maximum or minimum extend. In contrast, the sun’s light
intensity cannot be controlled since it is a natural factor not influenced by human beings. The relationship between
the forcing functions and state variables forms the core of an ecosystem model (Mitsch and Jorgensen, 1989).
Mathematical Equations...
Mathematical modelling provides a quantitative description of the ecosystem that must be modeled. With the use of mathematical models, the rates of the processes occurring in the ecosystem, and their stoichiometric interactions with the compounds, are formulated mathematically. The mathematical equations are used to represent the biological, chemical and physical processes occurring in the system of interest. Mathematical formulations should be incorporated in a solution procedure that considers the physical constraints and characteristics imposed by the system in which the processes are
taking place (e.g., temperature, mixing). The relation between the state variables and forcing functions can readily be depicted by using mathematical equations.
Parameters...
These are coefficients that can be found in mathematical representations of the ecosystem processes. These are considered constants for a specific ecosystem, or part of an ecosystem.
Modelling Procedures...
Ecological modelling, like any other modelling procedure, must undergo several critical steps prior to being accepted, as described in the following section.
Definition of the Problem...
The problem to be addressed and solved should be clearly defined. For the goals of this report, the task is to formulate a model that will include the interactions of algae and bacteria in the overall removal of organic carbon in secondary facultative ponds. All the selected forcing functions are used to evaluate the extent to which they influence the state variables, and the organic carbon removal process.
Model Selection...
This is a stage at which the conceptualization of a model, in the form of a diagram, is undertaken. It provides information on which state variables, forcing functions and process equations are required in the model. There are two steps involved.
A model may be theoretically developed that specifies the required variables and parameters, along with the associated equations. The second step is called numerical specification and validation, which requires the theoretically-developed
model to be run in a computer. At this stage, one may include, or reduce the bulkiness of the model, using scientific arguments.
Preliminary Simulations...
During this step, preliminary simulations of the system being modelled are performed. The procedure is useful for
identifying data deficiencies, theoretical gaps and the most important parameters.
Mathematical Equations...
The process of the oxidation of organic matter, the growth of biomass, and the influence of the forcing functions on the state variables, are linked together with the use of equations. Mathematical models describe the relationship between the model components, using specific mathematical expressions. The main concern in this step usually is the selection of the equation that correctly describes the processes. Figure 5.1 shows the necessary steps used for ecological modelling. The mathematical models proposed to describe the growth of microorganisms fall into two categories; namely structured and unstructured. The structured approach considers some aspects of the internal structure, and variations of the internal composition of microorganisms, and is important for the treatment of transient growth. The complexity associated with the structured models (i.e., large number of parameters) has limited their use to a few well-defined growth conditions. The unstructured models are based on a simplification of the complexity of living organisms, through the assumption that the amount of biomass present in the culture provides sufficient information about microbial activities (Tan et al., 1994).
The Monod (1949) equation is the most widely-used unstructured mathematical model for a single substrate-limited microbial growth.
Model Validation...
A model must be validated before it can be used with confidence for its intended purpose. This is done by running the calibrated model with a new set of data, with physical parameters and forcing functions to reflect new conditions. The
model is revised each time, until it is verifiably and consistently accurate. In contrast, the kinetic coefficients must
now be kept fixed at the values derived during the original calibration exercise. When the new model is validated, it
becomes an effective predictive tool for the range of conditions defined by the original calibration and validation data
set. If there is no match between predicted and real data, the model often can be analysed to determine the possible
reasons for the discrepancy.
Figure 5.1 A schematic diagram of the ecological modelling process, highlighting the needed information
Advantages of Modelling...
The process of modelling reduces the real world to a smaller world that may be more easily analyzed. If a model is made systematically, one may go back to the first step of model formulation, in order to increase the number of state variables
or forcing functions. It is possible in a modelling exercise to refine the conceptual model to fit the requirements of the formulated model. During the model calibration phase, it is possible to reduce the number of forcing functions and state variables, provided this gives a more accurate representation of the system being modeled. It is also possible to view the behavior of the model outcome, making it possible to change the values of coefficients in order to approximate the real outcome. Once a model is formulated, it can give a clear indication of the type of data to be collected from field studies for model calibration and validation. If one starts with data collection, and finishes with the model formulation, there
is no doubt that many data will not be useful for the model, often making it necessary to collect even more field data, thereby also making the first collected data set obsolete. Thus, a systematic understanding of the modelling procedure is
a fundamental requirement.
Models for Waste Stabilization Ponds and Constructed Wetlands...
The models for waste stabilization ponds and constructed wetlands are presented in Annexes 3 and 4. The model of organic carbon transformation in waste stabilization ponds is based on the growth processes of heterotrophic bacteria and algae
in the pond system. The forcing functions (temperature, light, etc.) are well used to predict the dominant processes in
the transformation of organic matter. The model on the transformation of nitrogen also was formulated, calibrated and validated. The coupled model of organic carbon in the primary facultative ponds and sub-surface wetlands also is presented
in Annex 4, followed by a model of nitrogen transformation in the horizontal sub-surface flow constructed wetland. The conceptual model and processes equations also are presented.