Stabilization Ponds - 1...

Introduction...
Stabilization ponds have been in use in the United States since 1901. Presently, there are approximately 7,500
stabilization ponds being used to treat wastewater and industrial wastes throughout the nation. These ponds (also
referred to as lagoons) can be used alone or in combination with other wastetreatment processes. Stabilization ponds
treat a wide variety of pollutants; therefore, it is imperative that they are constructed correctly to minimize the risk
of soil and groundwater contamination. Unfortunately, contamination occurs periodically and an understanding of groundwater hydrology and soil dynamics are needed to ensure effective remediation. This report will focus on the different types of stabilization ponds,their methods of wastetreatment,their construction and the potential hazards they pose to soil and groundwater if they leak.
Types of Stabilization Ponds...
There are four general types of wastewater ponds. All use microorganisms to degrade and detoxify organic and inorganic
constituents; the types of organisms differ among the four categories. Basic classification involves the description of
the dominant biological reaction that takes place in the pond. The four principle types are ;
- Facultative Ponds
- Anaerobic Ponds
- Aerated Ponds
- Aerobic Ponds
Facultative Ponds...
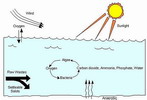
Facultative ponds are the most common form of wastewater lagoon. They are usually 1.2 to 2.5 meters in depth, with an
aerobic layer which overlies an anaerobic layer that usually contains sludge deposits. The crux of facultative operation
is oxygen production by surface reaeration and photosynthetic algae; the algae present one of the most serious problems associated with facultative ponds. These ponds require large land areas to maintain suitable BOD loadings. If structural failure occurs, a large area of an aquifer and soil are at risk of being contaminated.
Anaerobic Ponds...
Anaerobic Ponds have such a heavy organic loading that there is no aerobic zone. These ponds have average detention times
of 20 to 50 days. Two dominant biological reactions are acid formation and methane fermentation. These ponds are typically
used for the treatment of strong industiral and agricultural wastes and they tend to produce odorous compounds. Sodium nitrate and grease crusts are used to combat these odors. These compounds coupled with the acidic compounds formed through fermentation can be very damaging to soil and groundwater if the lagoon leaks.

Aerated Ponds...
In aerated ponds, oxygen is supplied through diffused or mechanical aeration. These ponds are generally 2 to 6 meters in
depth with detention times of 3 to 10 days and they are advantageous because they require very little land area.

Aerobic Ponds...
Aerobic ponds maintain dissolved oxygen throughout. They are typically 30 to 45 cm deep which allows sunlight to penetrate
at full depth. Detention time is usually 3 to 5 days. Because the detention time is so short, very little coliform
destruction will result. These coliforms pose a hazard to soil and groundwater purity if the lagoon leaks.

Processes of Stabilization Ponds and Potential Contaminants...
A major function of stabilization ponds is the removal of nutrients from wastewater and other waste by-products. Although
the effluent which comes from the lagoons has little nutrient content, the lagoon itself serves as a nutrient sink. The
high concentration of nutrients contained in the pond pose a risk to soil and groundwater as well. The high concentration
of nitrogen and phosphorous are examples of excess nutrients contained in many lagoons. Nitrogen can undergo a number of
physical and chemical processes which include ammonification, nitrification, denitrification and settling in organic
particulate form. Phosphorus is assimilated into algae cells within the pond. Both nitrogen and phosphorous become
potential contaminants to groundwater. Nitrogen is especially risky since nitrates, by-products of nitrogen decomposition,
are known to cause "Blue Baby Syndrome"; in infants who ingest water which contains excessive nitrate.
Wastewater Pond Design and Construction - USA Experiences...
 Two types of private onsite treatment systems are in general use in Kansas, septic tank-soil absorption systems and
wastewater stabilization ponds or lagoons. Where soil is sandy or loamy, the septic tank-soil absorption system is the
best choice. Where soil is clayey with poor drainage and space is available, wastewater ponds are often the best
alternative for treatment and disposal.
Two types of private onsite treatment systems are in general use in Kansas, septic tank-soil absorption systems and
wastewater stabilization ponds or lagoons. Where soil is sandy or loamy, the septic tank-soil absorption system is the
best choice. Where soil is clayey with poor drainage and space is available, wastewater ponds are often the best
alternative for treatment and disposal.
A wastewater pond is among the least expensive onsite treatment options, and maintenance is not excessive. A pond should
be the first consideration for wastewater systems where soils have severe limitations for soil absorption systems but are
well suited for pond construction and enough area is available to meet separation distance requirements. When soil, site
conditions, or closely spaced homes make a septic system or wastewater pond unsuitable, enhanced treatment methods such
as sand or media filter, rock-plant filter, mound, aerated tank, or other method should be considered. Surface discharge
for individual homes is not legal in Kansas.
This chapter presents design and construction information about wastewater ponds for sanitarians, contractors, owners, and
others. Operation, maintenance, and repair needs for wastewater ponds are presented in another bulletin. A wastewater pond
is a small fenced water body with a 3 to 5 foot liquid depth that receives sewage. Pond size is determined by the number of
occupants, size of home, the amount of wastewater, evaporation, and soil. Sewage enters the pond by a pipe below the surface
but above the bottom, near the center of the pond. Ponds must be nondischarging, meaning no overflow. Water is disposed of
by percolation and evaporation.
How Wastewater Ponds Work : A wastewater pond is a nutrient enriched complex ecosystem. Bacteria and other
microorganisms consume oxygen as they feed on sewage components and give off carbon dioxide used by algae. Unlike
other ponds, a wastewater pond's water should be green, because microscopic plants (algae) produce much of the needed
oxygen. Oxygen also enters as air blows across the water surface. Wastes are broken down by microorganisms into water,
gasses, and residual solids, which settle and accumulate in the pond. Properly-sized, carefully-constructed, well-operated,
and routinely-maintained ponds have no offensive odor. Although odor from a good pond is rare, when it occurs, it is
usually because the natural biological system is upset. Upsets can be caused by chemicals that disrupt the natural system,
organic overload from highly concentrated or too much waste, or accumulation of too much sludge. Extended cloudy weather
and spring or fall turnover also may contribute to temporary odor.
All trees should be at least 30 feet and shrubs 15 feet outside of the of embankment. Because sunlight is essential for
algae to produce oxygen, the east, south and west sides of the pond should not be shaded. Vegetation no taller than a 22
degree angle (2 ½ : 1 slope) from the berm is recommended. The minimum setback distance d in feet from the berm to plant
trees or shrubs that reach a height of h in feet is given by the formula d = 2 ½ x h. For example, a screen of lilac bushes
will reach 12 feet tall so they should not be planted closer than 2 ½ x 12 = 30 feet from the berm. In a properly sized pond,
solids are spread over a large area, so it should take at least 15 years before sludge removal is required. Tree leaves,
plant debris, or wildlife in or near the pond will contribute to faster sludge accumulation.
Wastewater Pond Location : A pond is best located down slope and away from the house so the sewer line to the pond
flows by gravity at the correct slope. When choosing the site, nuisance conditions, which could result from odors or
accidental discharge should be considered. Odors would least likely affect the owner when a pond is located northeast or
east of the house. Select an inconspicuous place 100 feet or more from the house and property lines, 50 feet from any
surface water, 30 feet from potable water lines, out of 100-year flood plain, and away from easements or rights-of-away.
Separation distances from surface water, wells, property lines, public water lines and such must be followed from local
codes or Kansas Department of Health and Environment (KDHE) Bulletin 4-2. A site plan showing all physical
features--surface and buried--and contour elevations will help to locate and design a pond. The bottom of the pond should
be at least 4 feet above highest groundwater level. The top of the pond embankment should be below the lowest drain or
clean out in the house. Sometimes the pond must be located upgrade of the house. This is more expensive because a pump
chamber and pump are required and pumps are also subject to failure. When pumping is required, it is advisable to add a
septic tank and have the system designed by an experienced person.
Phosphorus Removal...
Phosphorus (P) occurs in natural waters and in wastewaters almost solely as phosphates. These phosphates include organic
phosphate, polyphosphate (particulate P) and orthophosphate (inorganic P). Orthophosphates are readily utilized by aquatic
organisms. Some organisms may store excess phosphorus in the form of polyphosphates for future use. At the same time,
some phosphate is continually lost in the sediments where it is locked up in insoluble precipitates. Phosphorus is essential
to the growth of organisms and can be the nutrient that limits the primary use of a body of water. In the case where
phosphate is a growth-limiting nutrient, the discharge of raw or treated wastewater or industrial waste as well as
non-point source runoff to a body of water may result in the stimulation of growth of photosynthetic aquatic macro-and micro-organisms in nuisance quantities. As a result, there is a continuing effort to control the amount of P compounds
that enter surface waters in domestic and industrial discharges as well as non-point source runoff. With respect to
domestic wastewater, there are two means by which P is removed: chemical precipitation and the use of various biological treatment processes. In a lagoon treatment system, phosphorus is also removed by assimilation into the biomass of algae cells.
As the alkalinity increases during daylight hours, the phosphate is precipitated and settles out of the wastewater. Generally, the effluent P concentration is less than half of the influent wastewater concentration. Municipal lagoon wastewater treatment facilities which remove phosphorus by way of chemical addition are the subject of this special evaluation project (SEP). The purpose of this project is three-fold: (1) Evaluate the operating experiences of the
above referenced wastewater treatment technology; (2) Examine the degree of success of this type of treatment in removing phosphorus; and, (3) Identify operational problems. In order to obtain basic data for this project, thirty-two
municipalities in Michigan and Minnesota as well as respective State personnel, and the Ontario Ministry of Environment
were contacted.
Chemistry of Phosphorus Removal...
The lagoon treatment systems listed in the Appendix utilize the addition of chemicals to precipitate the P from the
wastewater. Chemicals typically used for P removal include metal salts such as aluminum sulfate (alum), and ferric
chloride. Ferrous chloride, lime, and various polymers are also used.
Aluminum Sulfate : The form of aluminum used for P removal is alum, a hydrated aluminum sulfate or
Al2(SO4)3. 14H2O. The chemical equation for the reaction of alum with
phosphate is as follows :
Al2(SO4)3. 14H20 + 2PO43- => 2AlPO4 + 3SO42- + 14H20
The factors that affect the actual quantity of alum required to attain a specific P concentration include alkalinity and
final pH of the wastewater, ionic constituents such as sulfate, fluoride, sodium, etc., quantity and nature of suspended
solids, microorganisms, and the intensity of mixing and other physical conditions extant in the treatment facility. The
optimum pH for P removal using alum ranges from 5.5 to 6.5, but in typical wastewaters, it ranges from 6.0 to 9.0.
Ferric Chloride : The chemical equation associated with the reaction of ferric chloride with phosphate is :
FeCl3 + PO43- => FePO4 + 3Cl-
Ferric chloride is most effective in removing P when the pH ranges from 4.5 to 5.0, with typical values of 7.0 to 9.0.
Canadian Experiences - An Example...
The International Joint Commission Report of 1969 resulted in the development and implementation of the Province of Ontario,
Canada policy requiring that the total P content in waste stabilization ponds (lagoons) be reduced to below 1.0 mg / L. Batch
chemical treatment of wastewater prior to discharge in seasonal retention lagoons was explored as one method of removing
phosphorus. In the early 1970's, the Ontario Ministry of Environment initiated a series of research projects on nutrient
control in sewage lagoons. The reports generated from these projects provided the baseline information upon which most
applications of this technology have been designed. Continuous and seasonal discharge lagoons were researched. Three
coagulants - ferric chloride, aluminum sulfate and lime - were field tested at various dosages. Ontario Province personnel
provided the manpower to handle chemical addition for these tests. The size of the lagoons was typically five acres and
above.
The chemicals were applied to and mixed into the wastewater of the secondary lagoon cell through the use of three 16 foot
aluminum or fiberglass boats equipped with a 100 or 150 gallon tank, chemical feed pump, and outboard motors. The pump
injects the chemicals into the propwash located at the stern of the boat. In distributing the chemical throughout the
lagoon, a grid-work pattern of boat travel was used. Boat speeds were adjusted to maximize the amount of turbulence
produced. The floc, formed by the chemical precipitants was given a minimum of 15 hours to settle out before lagoon
discharge began. The discharge period lasted from 1 to 15 days with the lagoon discharge cell being drawn down from six
feet to two feet or less.
The conclusions reached from these initial studies and long term experiences were - (1) batch chemical treatment of seasonal
lagoons achieved total P effluent of less than 1.0 mg / L; (2) effluent quality from batch treated lagoons was comparable to
or better than that achieved by conventional secondary treatment; (3) alum and ferric chloride applications produced
consistently high quality effluents while lime applications were not as effective in removing P; (4) outboard motorboat
method of application achieved good dispersal of the chemical and adequate mixing with lagoon wastewater; and (5) batch
chemical treatment is feasible for existing lagoon treatment systems which have adequate retention time for winter storage
and also is effective in removing algae from lagoon wastewater if the chemical dosage is sufficient. Based on these studies,
the Province of Ontario has designed and sucessfully operated over 20 full-scale municipal lagoon treatment systems using
alum to precipitate the phosphorus. These systems discharge on a seasonal basis (spring and fall).
Observations...
As stated above, operators of thirty-two municipal wastewater lagoon treatment systems with P removal in Minnesota and
Michigan as well as respective State Water Pollution Control Agency personnel, the Ontario Ministry of Environment, and
Regional office staff were contacted in order to ascertain specific basic operating data. This data was used to determine
the operating experiences as well as measure the success of these treatment systems in meeting P effluent limitation. The
following is a discussion of State specific observations.
Minnesota...
Design criteria has been established by the State which serves as a guide for consulting engineers in designing multi-cell
lagoon treatment systems. For primary cells, one acre of water surface should be provided for each 100-120 design
population. In addition, this cell should not exceed a BOD loading of 22 pounds/acre.day. The secondary cell(s) is utilized for storage and final settling and is designed at a minimum of one-third the volume of the entire lagoon system. The
storage capacity of the treatment system should be determined by both the average surface area and maximum operating depth
of all the cells. Typically, the cells should have sufficient capacity to store wastewater for a minimum detention period
of 180 days (covers the winter season and sufficient time for winter to summer transition). The cells of the lagoon treatment systems should be lined to retain the wastewater and to prevent its intrusion into groundwater. Normally, clay liners are a minimum of one foot thick. Other types of liners include vinyl and incorporated bentonite.
The State has eleven facultative lagoon wastewater treatment systems which utilize the addition of liquid alum directly
into the secondary cells via motorboat in order to meet the total P effluent limitation of 1.0 mg / L. These treatment
systems have design flows ranging from 0.017 to 0.672 million gallons per day (mgd) with permitted seasonal (spring and
fall) discharge. The years of operation of these treatment systems ranges from 1 year to 7 years. The procedures used
for addition of alum are very similar to those utilized in Ontario, Canada. The alum is delivered in liquid form (tanker
truck) or dry form (bags) and is stored on-site. It should be noted that prior to application, the dry alum is mixed with
water to form a solution. The alum is applied to the secondary cell by way of two methods. Both methods utilize a 12 to
17 foot boat equipped with a storage tank (55 to 500 gallon size), chemical feed equipment, and an outboard motor ranging
in size from 5 horsepower (hp) to 50 hp. In a majority of these cases, the alum is fed into propwash and is mixed as a
result of the action of the outboard (propeller driven) motor. However, in two cases, the alum is sprayed onto the wastewater via outriggers on both sides of the boat. The latter method, though ensuring full surface coverage, would appear to not as thoroughly mix the alum with wastewater as would applying the alum through the propwash, though adequate P removals are achieved.
The operators use two methods for determining the appropriate alum dosage. One method involved the operator determining
the phosphorus concentration in the lagoon cells and matching the reading with those in a precalculated chart. This chart
lists the associated alum dosage which would be applied to the lagoon wastewater at the level of phosphorus concentration
obtained in the sample. Then the dosage amount is applied in the lagoon cell(s). The other known method involved the use
of past experiences of applying alum on the part of the operator. Should conditions change (e.g. changes in phosphorus
concentration), the operator will either add more or less alum to ensure continued compliance with P effluent limitations.
The P-influent values for the eleven treatment systems in Minnesota ranged from 1.5 mg / L to 6.0 mg / L with the average
being approximately 3.3 mg / L. The effluent levels for P for all these systems regularly met the 1.0 mg / L effluent
limitation. There were several minor excursions (10 percent) above the limit but no pattern or specific cause was
discovered. The only exception was one facility with an infiltration problem which required discharge in the middle of
winter when the pond surface was frozen.
Michigan...
Operators in Michigan have used a somewhat different application of this technology at over 26 municipal lagoon treatment
systems currently in operation. The years in which these treatment systems have been in operation ranges from 1 year to
over 20 years. hese include aerated as well as facultative lagoons with the majority constructed with 3 to 6 lagoon cells.
These included not only systems designed for seasonal discharge (once or twice a year), but also, continuous discharge
systems (varying from 24 hours / day, 7 days / week to 8 hours / day, 5 days / week), as well as continuous discharge
lagoons where the chemicals are added to a clarifier following the lagoon system. The sizes range from 0.25 to 7.5 mgd.
None of these facilities use motor boats to add the chemicals to the lagoons, but rather, they typically rely on a mixing chamber located between the lagoon cells and clarifier. Chemicals are added continuously or more specifically, whenever wastewater is flowing through the mixing chamber. The P influent values for the 21 treatment facilities in Michigan ranged from 0.5 mg / L to 15.0 mg / L with the average being approximately 4.1 mg / L.
A wide variety of chemicals is used including ferric chloride and alum. In addition, different polymers are used in conjunction with the metal salts. A few treatment systems even have the flexibility to add alum or ferric chloride alternately. The permit limitations generally are written with a 1.0 mg / L effluent maximum based on a 30-day average but several are based upon a pound per day maximum value or 30-day average pound per day value. The State does not have specific guidance for designing wastewater treatment lagoons. However, as a minimum, the State advises consulting engineers to use
the criteria discussed in the Recommended States Standards for Sewerage Works (i.e., Ten State Standards). In addition,
the State recommends that lagoon cells should be lined with either compacted clay or synthetic liners with protective
soil cover for protection of groundwater.
Tests for Total Phosphorus...
Generally, phosphorus analysis has two procedural steps - the conversion of the phosphorus form of interest to dissolved
orthophosphate, and the colorimetric determination of dissolved orthophosphate. Two types of analysis (tests) for total
phosphorus are being utilized in Minnesota and Michigan. Detailed information on both tests is given in the fourteenth
edition of the "STANDARD METHODS (1993)". The ascorbic acid test, which is the EPA-approved test for total phosphorus is
most useful for routine wastewater samples below 1.0 mg / L of phosphorus. The apparatus used for this test includes
colorimetric equipment, a spectrophoto-meter, a filter photometer, and acid-washed glassware. Care needs to be taken when
conducting this test due to its sensitive nature in terms of time. The reagents are sulfuric acid, potassium antimonyl
tartrate solution, ammonium molybdate solution, ascorbic acid, and standard phosphate solution. The principle behind this
test is the reaction of ammonium molybdate and potassium antimonyl tartrate with orthophosphate in acid medium to form
heteropoly acid hosphomolybdic acid. This acid is reduced to an intensely colored molybdenum blue by the ascorbic acid.
Vanadate method or the vanadomolybdophosphoric acid colorimetric method is useful for wastewater samples in the range of
1 to 10 mg / L of phosphorus. The reagents are the standard phosphate solution, hydrochloric acid or sulfuric acid,
phenolphthalein indicator, potassium sulfate, and the vanadate-molybdate reagent. The apparatus a spectrophotometer,
colorimetric equipment, a filter photometer, acid-washed glassware, filtration apparatus, and filter paper. The general
principle behind this test is the formation of a heteropoly acid, molybdophosphoric acid resulting from the reaction of
ammonium molybdate in a dilute orthophosphate solution under acid conditions. Yellow vanadomolybdo- phospheric acid is
formed in the presence of vanadium. The intensity of the yellow color is proportional to phosphate concentration. The
color remains stable for several days and its intensity is unaffected by room temperature variations. This method is the
easiest to conduct for total P and is less sensitive than the ascorbic acid test.
Conclusions...
The overall experience with these systems is that the technology, in its various configurations, has been working very well.
Of the thirty-two lagoon treatment facilities reviewed as part of this report, only two facilities are considered to be in
significant noncompliance, though one of these is not due to the technology but rather to excessive clearwater entering the
system resulting in discharges outside of the spring and fall permitted discharge. The other facility in noncompliance has
identified a problem with resolebilization of precipitated phosphorus that the operator believes is related to a change in
pond pH caused by algal blooms. The chemical equilibrium of precipitating phosphorus with metal salts is pH dependent but
none of the other facilities seemed to have experienced this phenomenon. Most of the facilities though did report typical
lagoon operating problems. These included seasonal algae blooms which are a common source of total suspended solids in the
effluent, and mixing of surface wastewater, algae, and duckweed which results in the resuspension of precipitated solids as
well as an increase in biological oxygen demand (BOD) and suspended solids (SS). Other typical problems were associated
with the handling, storage and mixing of the chemicals which were discussed in the above referenced EPA documents.
A number of minor instances have occurred at some of the lagoon treatment systems where the actual effluent P value exceeded
the P effluent limitation (less than 10 per cent). Many of the Regional treatment systems do a minimum of process control
testing for adjustment of the chemical dosage. Often, they rely on experience gained from discharges of past years, adjusting the dosage in steps rather than by recalculating dosage based upon phosphorus levels in the pond. Whether these minor permit violations are a result of minimizing operator time at the facility, laboratory costs, and chemical dosing or inadequate operation and maintenance could not be readily determined. This varies somewhat from the Ontario experiences
which depends upon a larger dose of chemical to ensure permit compliance as well as reap a secondary benefit of discharging less phosphorus (and BOD and SS that is also precipitated) to the receiving waters.
None of these lagoon systems experienced problems with buildup of sludges to levels which affected the effluent
concentrations. There were a few problems noted with localized sludge accumulation within the lagoon. Accumulated
amounts were an inch or less per year, consistent with solids buildup in the primary lagoons cells. None of the Ontario lagoons have had to remove sludge, but several did as part of lagoon expansion projects. Chemical addition on a continuous
or batch basis is easily calculated and applied through an influent structure or via motorboat. The systems can and have regularly achieved effluent total phosphorus limits of 1 mg / L or less under a wide variety of lagoon configurations, climatic conditions, and a wide range of design flow rates (0.25 to 7.5 mgd). The secondary benefits of chemical precipitation result in lower BOD and SS levels in the effluent lagoon, which can partially counteract or overcome the variability of algae and other suspended solids in the lagoon effluent resulting in a more consistent permit compliance.
The addition of chemicals to an existing lagoon via motorboat or mixing structure requires a relatively low capital investment and operates quite well within many existing lagoon configurations. It should be noted that as with any
wastewater treatment system, the type of treatment system discussed in this report depends upon the operator's time, knowledge, and attention to ensure its proper operation and maintenance.
Pathogenic Bacteria...
Pathogenic organisms such as Shigella, Salmonella, Escherichia, Leptospira, Vibria and Francisella are contained in
some lagoons. Viruses and protozoa also exist in abundance in these ponds. Water is not the natural habitat of these organisms but it serves as a means of transport to new hosts. Although pathogenic organisms are usually unable to survive
or multiply for long periods in water, studies have shown that viable numbers of organisms remain present in some lagoons
at all times. This becomes a particular concern for lagoons which are located near high water tables that merge into
nearby streams and reservoirs.

Pond Sealing...
In order to prevent contamination of groundwater, pond seepage must not be able to occur. Therefore, pond sealing is a primary requirement in the successful design, construction, and maintenance of a lagoon. Pond sealers fall into three categories: (1) cement liners, (2) synthetic and rubber liners, and (3) chemical and natural treatment sealers.
Cement Liners...
Cement liners can be constructed of asphalt and montmorillonite clay as well as cement. The usual construction of a
cement liner involves the preparation of a gravel bed approximately 15 cm deep followed by the application of a clay,
cement, or asphalt layer which settles through the gravel and seals the voids. This forms a formidable barrier to pond seepage. The small amount of seepage that can occur is usually 0.20 - 0.25 m3 / day. It is important to note
that clay tends to be the best primary liner in this category because it shrinks and swells according to variable temperatures and wet-dry conditions. Cement and asphalt liners can crack under such conditions.
Synthetic Liners...
Synthetic liners are the most popular of all liners because seepage is impossible if they remain unpunctured and
uncorroded; therefore, it is important for the synthetic liners to be laid cautiously and properly during their
installation. Synthetics are available in large sheets, they're chemically inert and economical to install. These
liners are particularly useful in ponds which contain toxic wastewaters. When used in conjunction with cement liners, synthetic liners allow no seepage.
Natural Liners...
Natural liners are formed by the physical clogging of soil pores by settled solids, biological clogging caused by microbial
growth at the pond lining, and chemical clogging of soil voids due to ionic exchange. This method of pond sealing is haphazard and unreliable because the dominant mechanism of the three natural sealants depend upon the characteristics of
the wastes being treated.
Factors Affecting Liner Performance...
While most liners perform satisfactorily for an average of 15 years, premature failure usually results from 1 or more of
the following factors ;
- Membrane puncture
- Weed growth
- Placement of lining on unstable side slopes
- Scour of cover material
- Surface Runoff
- Cleaning operators
- Substandard liner materials
- Inadequate protection of the membrane
It is important to keep these factors in mind when designing, constructing and maintaining lagoons. Most states set guidelines regarding the factors aforementioned. It is imperative that an owner/operator of a lagoon meet the prescribed specifications of that state; if the owner fails to meet these requirements and the lagoon contaminates it's surroundings, he/she can be fined heavily by the state DEQ and the EPA. Described below are the general guidelines set by the Virginia Department of Environmental Quality for lagoon design construction and maintenance.
General Guidelines Regarding Lagoon Design, Construction, and
Maintenance Set by the State of Virginia...
All surface lagoons must be designed with a liner on all portions of the impoundment. The liner must be intended,
constructed, and installed to prevent any flow of wastes out of the lagoon into the subsurface soil or groundwater
during the entire life of the lagoon. The liners must be placed upon a foundation capable of providing support to the
liner. The foundation must provide resistance to pressure gradients above and below the liner to prevent failure due to settlement, compression or uplift. The operator of the lagoon must install a leachate collection system. The lagoon must
be designed and constructed to prevent overtopping resulting from wind, excessive rainfall, malfunction of the level controller, and human error. The operator must demonstrate that the lagoon is located, designed, and operated in a manner which does not allow migration of waste into the groundwater or surface water at anytime. During lagoon construction, the liner must be inspected to ensure tight seams and joints and the absence of tears and punctures. The soil based and admixed liners must also be inspected for imperfections. After construction of the lagoon is complete, a certified engineer must examine the lagoon to ensure that the lagoon will operate according to it's design specifications.
During operation of the lagoon, weekly inspections of the lagoon need to be made. Lagoons should also be checked for
structural integrity after every major storm. The lagoon should be removed from service if the contents in the lagoon suddenly drop for no reason. If a leak is responsible for the drop in effluent level, it must be located and plugged immediately. If the leak can not be located, the operator must remove the waste from the lagoon and inspect the liner. If
the hole can not be patched and if the leak remains, the lagoon must be decommissioned. When the surface impoundment can
no longer be used, the removal of all the waste, liner, and any contaminated soil must be removed from the site. The waste will be treated as either solid or hazardous waste.
Dynamics of Lagoon Failure and Subsequent Contamination of
Soil and Groundwater...
When a surface impoundment fails, soil and groundwater contamination is likely to occur. If the lagoon contains animal
wastes, the pollutants tend to be nitrates, phosphorous and fecal coliforms. Phosphorous is usually sorbed to soil particles rather quickly; however, nitrate leaches readily and can pose serious threats to human health in some instances (see
Potential Contaminants). The transport of fecal coliforms into groundwater is limited by the porosity of the soil matrix; transport is easiest through sand and most difficult through clay. The extent of contamination from animal waste storage ponds depends upon the location of the water table. If the surface impoundment of a lagoon containing chemical or hazardous waste fails, the pollutant can be sorbed to soil particles or dispersed in groundwater; this is dependent upon the porosity of the soil matrix, the depth to groundwater, the partition coefficient of the waste and it's chemical structure.
Regulations...
General Requirements...
Waste stabilization ponds may be used if designed and constructed in accordance with the following criteria and provided
the effluent is discharged in accordance with the requirements of the general NPDES permit fisted in rule 69.2(455B). A
septic tank sized according to rule 69.5(455B) shall precede a waste stabilization pond.
Location...
Waste stabilization ponds must meet the following separation distances :
- 1,000 feet from the nearest inhabitable.residence, commercial building, or other inhabitable structure. If the
inhabitable or commercial building is the property of the owner of the proposed treatment facility, or there,is written
agreement with the owner of the building, this separation criterion shall not apply. Any such written agreement shall be
filed with the county recorder and recorded for abstract of title purposes, and a copy submitted to the department.
- 1,000 feet from public shallow wells.
- 400 feet from public deep wells.
- 400 feet from private wells.
- 400 feet from lakes and public impoundments.
- 25 feet from property lines and rights-of-way.
Size...
Dimensions : Ponds shall have a length not exceeding three times the width.
Capacity : When domestic sewage from a septic tank is to be discharged to a waste stabilization pond, the capacity
of the pond shall be equivalent to 180 times the average daily design flow.
Depth : The wastewater depth for a waste stabilization pond shall be uniform and 3 feet to 5 feet.
Freeboard : A minimum freeboard of 2 feet shall be maintained at all times.
Embankments...
Seal : Embankments shall be constructed of impermeable materials and shall be compacted. The bottom of the waste
stabilization pond shall be cleared and leveled to the required elevation and shall be lined with an impermeable natural or
man-made material. Seepage loss through the sides and bottom shall be less than 1/16 inch per day.
Slopes : Inside embankment slopes shall be 3 horizontal to 1 vertical. Outside embankments shall be at least 3:1.
Berm top : Berm tops shall be at least 4 feet wide.
Cover : Embankments shall be seeded from the outside toe to the inside high water line. From the high water line
down the embankment diagonally about 5 feet shall be rip-rapped for erosion and vegetation control.
Inlet and Outlet Structures...
Inlet : The inlet shall be placed no higher than 12 inches above the bottom of the pond. It shall discharge near the
middle of the pond at a point opposite the overflow structure and onto a concrete splash plate at least 2 feet square.
Outlet : The outlet pipe shall withdraw water from a submerged depth of at least 1 foot. The intake for the outlet
pipe shall be 3 to 5 feet from the embankment.
Separation : The inlet and outlet should be separated to the maximum extent possible, ideally by a berm or baffle
constructed in the lagoon to prevent short-circuiting.
Drainage...
All surface water shall be diverted away from the waste stabilization pond.
Discharge...
Controlled discharge : If the pond is designed for open discharged, it must be discharged under controlled
conditions. The effluent must be tested before discharge, and effluent quality must be less than 25 mg / L of
BOD5 and less than 25 mg / L of TSS. Another test must be taken during discharge with the same results. Pond discharge is permitted only in spring and fall when stream flows are highest.
Continuous discharge : If the pond is to have an unlimited continuous discharge, the effluent shall receive
additional treatment through the use of intermittent sand filters, mound systems or subsurface absorption systems of a magnitude of half that prescribed in rules 69.6(455B), 69.7(455B) and 69.9(455B). Under continuous discharge, effluent sampling shall be as required for constructed wetlands as outlined in 69.1 1 (1) c.
Maintenance...
Fencing : All waste stabilization ponds are to be fenced adequately to prevent entrance of livestock and to
discourage entrance by people into the area. Signs shall be posted warning of possible health and safety hazards.
Vegetation : Vegetation on the top and sides of the berm shall be kept mown. No trees shall be allowed to become established.
Wastewater Treatment Ponds...
Wastewater treatment using ponds can be an economical treatment method which produces a highly purified effluent. The
degree of treatment provided depends on the type and number of ponds used. Ponds can be used as the sole type of treatment
or they can be used in conjunction with other forms of wastewater treatment. Ponds can be named or classified based upon their location in the wastewater treatment process and the type of wastes they receive.
Raw Sewage Stabilization Pond...
This is the most common type of pond. Receives wastewater which has received no prior treatment (except screening or shredding). Designed to provide a minimum of 45 day detention time and to receive no more than 50 pounds of
BOD5 per day per acre. Normal operating depth of the pond is 3 - 5 feet. The quality of the discharge is
dependent on the time of the year. Summer months produce high BOD5 removals but low suspended solids removals. Winter months have poor BOD5 removal but excellent suspended solids removals. Consists of an influent structure, pond berm or walls and an effluent structure designed to permit selection of the best quality effluent. Processes occurring in the pond include settling, aerobic, facultative and anaerobic decomposition and photosynthesis to produce required
oxygen.
Oxidation Pond...
Receives flows which have passed through a stabilization pond or primary settling tanks. This type of pond provides biological treatment, additional settling and some reduction in the number of fecal coliform present. Normally designed
using the same criteria as the stabilization pond.
Polishing Pond...
Receives flow from the oxidation pond or from other secondary treatment systems. Process removes additional
BOD5, solids and fecal coliform and some nutrient removal. Polishing ponds are designed to provide 1 to 3
days detention time and normally operate at a depth of 5 to 10 feet. Excessive detention time which increases effluent suspended solids concentrations.
Ponds may also be classified or named by the type of processes occurring with in the pond.
Aerobic Pond...
Oxygen is present throughout the pond. All biological activity is aerobic decomposition. Not widely used.
Anaerobic Pond...
Normally used to treat high strength industrial wastes. No oxygen present in the pond. All biological activity is anaerobic decomposition.
Facultative Pond...
Most common type of pond based upon processes occurring. Oxygen is present in the upper portions of the pond and aerobic processes are occurring. No oxygen present in the lower levels of the pond. Processes occurring in the lower levels are anoxic or anaerobic. The facultative stabilization pond involves many physical, chemical and biological processes. The
key to operation is the balance between photosynthesis and aerobic decomposition.
Aerated Pond...
Oxygen is provided through the use of mechanical or diffused air systems. When aeration is used the depth of the pond
and/or the acceptable loading levels may increase. Mechanical or diffused aeration systems may be used to supplement
natural oxygen production or to replace it.











