|
Properties
|
Nostocoida limicola I
|
Nostocoida limicola II
|
Nostocoida limicola III
|
|
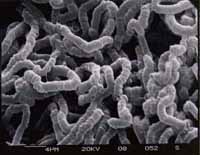
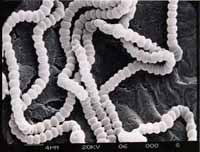
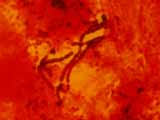
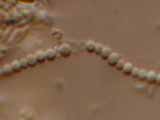
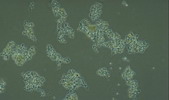
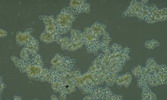
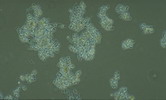
Micrograph
|
|

|
|
|
Microscopic details
|
Nostocoida limicola I, Gram stain x 1,000, bright field.
|
Nostocoida limicola II, Nomarski Phase Interference x 1,250.
|
Nostocoida limicola III, Nomarski Phase Interference x 1,250.
|
|
Cell Properties
|
|
Size
|
0.6-0.8 Ám diameter.
|
1.0-1.4 Ám diameter.
|
1.6- 2.0 Ám diameter.
|
|
Shape
|
Spherical to oval or disc-shaped.
|
|
Septation
|
Present but not easily seen.
|
Clearly visible.
|
|
Inclusions
|
Absent.
|
PHB granules.
|
|
Motility
|
Non motile.
|
|
Trichome Properties
|
|
Length
|
Short <200 Ám. Bent or coiled.
|
|
Location
|
Extending from flocs, within flocs and free in bulk liquid.
|
|
Attached growth
|
Growth seldom seen.
|
Absent.
|
Rare.
|
|
Branching
|
Absent.
|
Incidental branching reported.
|
Rare.
|
|
Sheath
|
Absent.
|
|
Staining Reactions
|
|
Gram stain
|
Positive.
|
Variable, often negative.
|
Positive.
|
|
Neisser stain
|
Negative.
|
Variable, often negative.
|
Positive.
|
|
Diagnostic features
|
Trichome morphology with spherical cells.
|
Trichome morphology with spherical cells. Larger than N. limicola I.
N limicola II is the most commonly observed morphotype.
|
Trichome morphology and cell size, larger than N. limicola I and II.
"Poppet- bead" appearance. Staining response.
|
|
Ecology / Occurrence
|
|
Often found incidentally in foams and mixed liquors with N. limicola II occasionally dominating in bulking plants. In Australia, more commonly seen than in other countries surveyed. The
distinction between N. limicola I, II, and III is on their cell diameter. Found in underloaded plants. Controlled by anoxic selector. Washout at cell residence time < 4 days.
|
|
Physiology
|
|
Only few strains examined in pure culture and these were probably chemoheterotrophs and facultative anaerobes. N. limicola II grew well on a range of carbohydrates aerobically and anaerobically, if complex Nitrogen source like peptone present. Optimal growth at 23- 28C. Little data on their
physiology in plants.
|
|
Taxonomy
|
|
N. limicola II is a member of the Arthrobacter group of the Actinomycetates (Blackall et al, 1995) but the relationship between N. limicola I, II and III is unknown.
|
|
Other
|
|
Considerable confusion exists since the descriptions of these organisms in
the literature vary. The ability to culture them should encourage further physiological and taxonomic work.
|







 "A Good Floc Forming"...
"A Good Floc Forming"...


 "Bulking Sludge Forming"...
"Bulking Sludge Forming"...













 "Floc Interactions"...
"Floc Interactions"...