|
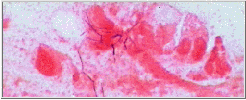
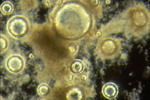
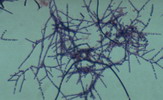
"Microthrix parvicella"
|
M.P. : Long coiled unbranched Gram positive filaments. A major cause of foaming in Europe, Australia and South Africa, but less common in published reports from USA. More prevalent in cooler climates.
|
|
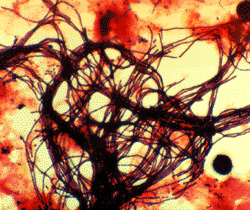
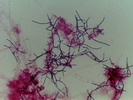
"NALO" or "GALO"
|
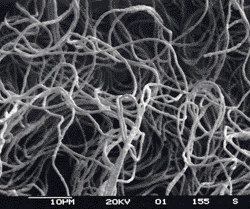
Nocardia : Shorter branched filaments with branches at approximately right angles (so called "Nocardia" or Nocardia amarae-like organisms (NALO), but since N.amarae has recently been reclassified as Gordona amarae these are now sometimes called Gordona amarae like organisms or GALO). All are members of the mycolata (mycolic acid producing organisms) and may belong to genera such as Nocardia, Gordona, Rhodococcus, Tsukamurella, Dietzia and Mycobacterium.
|
|
"PTLO"
|
Nocardia pinensis and Skermania piniformis : The only "Nocardia" with morphology sufficiently different to allow identification in its own right. Originally called PTLO (Pine Tree-Like Organism) because of its tree-like branching morphology. Now classified as Skermania piniformis. Member of the mycolata.
|
|
"Actinomycetes"
|
Gram positive branched rods without the distinctive branching patterns of NALO and Skermania piniformis. This description includes coryneform bacteria. Probably members of the mycolata.
|
|
"Gram positive cocci"
|
Found in non-filamentous foams in Australia. May be members of the mycolata, including coccal stage of Rhodococcus and Nocardia farcinica.
|
|
"Eikelboom Type 0675"
|
Type 0675 was significant in recent French studies. Some studies don't differentiate between Types 0041 and 0675.
|
|
"Eikelboom Type 0092"
|
More common in nutrient removal plants. Its role as a foam-former is disputed.
|
|
"Nostocoida limicola"
|
Recent surveys suggest this is becoming more common as a foam former.
|
|
"Eikelboom Types 0803, 0413, 1851, 021N, 0914, 0581, 1863, 1701, Haliscomenobacter hydrossis, Sphaerotilus sp, Acinetobacter sp and Cyanobacteria"
|
Occasionally reported as the dominant organism in foam, but their incidence is low.
|
 "Denitrification"...
"Denitrification"...
 "Denitrification"...
"Denitrification"...