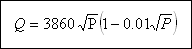Water Supply...
Water Supply Sources...
The primary source of water is rain and as it falls on the ground a certain portion percolates into the ground, a certain flows into rivers, streams, etc. The melting of snow on the peaks of high mountains causes sometimes floods in the rivers. The rainwater, which is soaked by the ground, forms the sources of groundwater supplies and the portion, which flows in streams, forms the surfacewater supplies.
The groundwater percolates through pores of soil. The earth is formed of different layers of materials like sand, clay, gravel, limestone, etc. The layers like those of sand, gravel, etc actually allow the water to pass through them and they contain quantities of water in them. These are called aquifers or water-bearing strata. The other layers like limestone, sandstone, etc, which do not allow water to pass through them, are called impervious strata. The pervious and impervious strata are found alternatively. The top layer of earth is usually pervious.
( 1 ) Ground water...
Water which is contained by an impermeable layer which will flow to wells, springs, or other points of recovery is
called groundwater. Groundwater supplies are much more widely distributed than surface supplies. The ground water
sources may be of three types, (i) springs (ii) wells (iii) infiltration wells and galleries.
(i) Springs : An opening in the ground surface from which groundwater flows is a spring. Water may flow by force
of gravity (from water-table aquifers), or be forced out by artesian pressure. The springs may be of two types ; gravity
springs and artesian springs. The flow from a spring may vary considerably.
(a) Gravity springs : These are formed when the underground watertable gets exposed on the slopes of hills etc. The water-bearing stratum overlying an impervious stratum like rock or clay. The spring is formed at the junction of these strata.
(b) Artesian springs : These are formed under certain geological conditions. The inclined or basin shaped water bearing strata (in between two impervious strata) are exposed to the surface on the higher side in this case. The rain water
flowing on the surface enters into this basin (poros stratum) through the exposed portions on the top, flows down and is finally stored between the two impervious layers under hydrostatic pressure. Now, if a hole is bored right up to this
water bearing peculiar shaped stratum, water rushes upwards sometimes above the surface in the form of a fountain.
(ii) Wells : A well can be used to extract water from the groundwater reservoir. Pumping will cause a lowering of
the water table near the well. The wells are also classified as : (a) shallow well and (b) deep well. One should clearly understand that these terms have nothing to do with the actual depths of the wells. Shallow well may sometimes be deeper
than deep wells.
(a) Shallow wells : These are wells dug in the uppermost layer of earth and obtain their water supply from the subsoil watertable. These are very suitable for supplying water in rural areas or for only a portion of a town as the discharge is limited to about 4 l/s. The quality of water in this case is not good as the water is collected by the percolation and may get discharges from soak pits etc.
(b) Deep wells : Deep wells are those which are drilled to an aquifer before an impervious stratum like clay. As the water travels a long distance from the outcrop to the site of the well, it gets purified due to natural filter of soil articles. The water is usually hard as it contains dissolved salts in it.
(iii) Infiltration wells and galleries : The infiltration wells and galleries are of importance for country, which
the layer of sand and porous alluvium is available up to at least 2 m depth in the bed of the river. So far as the quality
of water in this case is concerned, there is no need of treating it in the filter beds because the water already trickles down the bed of the sand. Disinfection is however necessary. These are three infiltration wells, which are joined by porous pipes. The filtered water from all these wells is carried to an inspection well and finally to a jack well which is provided on the top of the bank in which a pump is also installed.
It consists of a tunnel made up of concrete or masonry and floor of cement concrete. The walls are constructed with
dry stones or with stone masonry or with brick-in-lime masonry or with concrete. Numerous weep holes are provided. The
entire upper surface of the gallery is covered with 1.0 m ruble, gravel and sand.
The infiltration galleries are cheap and dependable. Where surface water in a perennial stream does not meet the water
demand in dry weather, infiltration galleries can constitute a good source of water. In case these are not properly
located and maintained, these may choke and washed away due to heavy flood. Therefore, proper investigations including reconnaissance survey of the areas of probable siting of the galleries or the wells should be conducted. The boring should
be done preferably up to first impervious layer at 15 m interval to furnish the data necessary to judge the feasibility
of such a scheme. The studies regarding change of course of stream, physical and chemical characteristics of water during different seasons should be conducted.
( 2 ) Surface Water...
Precipitation that does not enter the ground through infiltration or is not returned to the atmosphere by evaporation flows over the ground surface and is classified as surface water or direct runoff. The surface water is available in the form of rivers and streams, natural lakes formed by impounding reservoir by dam or bounds, rain water from roofs, etc. Generally,
the water supplies are derived from streams. The runoff depends upon the geographical feature of the catchments, topography, soil, geological structure, storage in the catchments, temperature, character of rainfall and amount of rainfall, etc. It is 20 50 %. The quality of surface water depends upon the nature of soil through which they traverse. In the upland regions, the water is generally soft and in the low land regions it is hard. The surface water becomes more hard in the dry season. The flood water may be polluted due to organic and inorganic wastes.
( 3 ) Snow...
Much of the snow falling on a watershed is kept in storage on the ground surface until temperatures rise above freezing. In the mountainous areas of the western United States, snow storage is an important source of water supply trough much of the normal irrigation season. Measures taken to increase the snowpack and reduce the melt rate are usually beneficial to individual water supply system in theses areas.
( 4 ) Saline Waters...
Saline waters include (a) undiluted seawater containing about 35,000 mg/L total dissolved solids (TDS) and (b) brackish
water containing from 1,000 mg/L to about 15,000 mg/L TDS.
( a ) Seawater : Seawater is available at sea level and at the seacoast. It is important as a major water resource because of the size and importance of coastal communities that can be served by converted seawater.
( b ) Brackish water : Sources of brackish water are less obvious and less well known than those of seawater. They
are less expensive to treat than seawater. Brackish water is found underground as well as in estuaries, rivers, lakes and certain wastewaters.
(i) Groundwater : The most wiely available and in many ways the most desirable source of brackish water is brackish groundwater. Brackish groundwater reserves are found in many parts of the world, including US, Canada, Mexico, Middle
East, Australia, southern and western Europe.
(ii) Estuaries : Estuaries of some major rivers extend inland for many miles and represent major sources of brackish
water. Practical salt-removal processes give water engineers a new degree of freedom in dealing with estuaries water problems.
(iii) Rivers and lakes : A few major rivers and large lakes are saline or brackish in nature. Attempts are being made to reduce the salinity of some of these streams by control of natural salt pollution sources.
(iv) Wastewaters : Three of the major beneficial uses of water result in substantial increases in its mineral
content-namely irrigation, industrial cooling by evaporation, and municipal use.
Selection of the Water Supply Source...
Both the surface and underground water may be utilized for the public water supply schemes. The selection of a particular source depends upon its permanency, adequacy and the cost of the scheme. Each source will require different types of treatment of water depending upon the quality of water of the source. For example, the river water requires to be
completely treated before use whereas for well water an elaborate treatment is not necessary. Thus, the selection of a particular source depends on local conditions. In hilly areas, the springs may prove to be a good source and for a small colony a well may be suitable source.
Quality of Water Sources...
Water supply development is concerned with both the quantity and quality of water required to meet the needs of man in an efficient and economical manner. Neither factor can be neglected. The usefulness of the maximum available water supply is determined in large part by its quality.
Groundwater quality is influenced considerably by the quality of the source. Changes in sources waters or degraded quality
of normal supplies may seriously impair the quality of the groundwater supply. Municipal and industrial wastes entering an aquifer are major sources of organic and inorganic pollution. Large scale organic pollution of groundwaters is infrequent, however, since significant quantities of organic wastes usually cannot be easily introduced underground. The problem is
quite different with inorganic solutions, since these move easily through the soil and once introduced are removed only
with great difficulty. In addition, the effects of such pollution may continue for indefinite periods since natural
dilution is slow and artificial flushing or treatment is generally impractical or too expensive. The number of harmful enteric organisms is generally reduced to tolerable levels by the percolation of water through 6 or 7 ft of fine-grained soil. However, as the water passes through the soil, a significant increase in the amounts of dissolved salts may occur. These salts are added by soluble products of soil weathering and erosion by rainfall and flowing water. Locations
downstream from heavily irrigated areas may find that the water they are receiving is too saline for satisfactory crop production. These saline contaminants are difficult to control because removal methods are exceedingly expensive. A
possible solution is to dilute with water of lower salt concentration (wastewater-treatment plant effluent, for example)
so that the average water produced by mixing will be suitable for use.
The primary causes of deterioration of surface-water quality are municipal and domestic wastewater, industrial and agricultural wastes (organic, inorganic, heat), and solid and semisolid refuse. A municipality obtaining its water supply from a surface body may find its source so fouled by wastes and toxic chemicals that is unsuitable or too costly to treat
for use as a water supply. Fortunately, waste products discharged by cities and industry can be controlled at the point of initiation.
Quality of Water Supplies...
Precipitation in the form of rain, snow, hail, or sleet contains very few impurities. It may entrain trace amounts of
mineral matter, gases and other substances as it forms and falls through the earths atmosphere. The precipitation,
however, has virtually no bacterial content.
Once precipitation reaches the earths surface, many opportunities are presented for the introduction of mineral and
organic substances, microorganisms, and other forms of pollution (contamination). When water runs over and through the
ground surface, it may pick up particles of soil. This is noticeable in the water as cloudiness or turbidity. It also picks up particles of organic matter and bacteria. As surface water seeps down into the soil and through the underlying material
to the water table, most of the suspended particles are filtered out. This natural filtration may be partially effective in removing bacteria and other particulate materials; however, the chemical characteristics of the water may change and vary widely when it comes in contact with mineral deposits. Chemical and bacteriological analyses can be performed by a state or local health department or by a commercial laboratory. A wide variety of analytical methods are used to characterize
drinking water quality to meet regulatory requirements and to evaluate performance at each step of a treatment process.
The widespread use of synthetically chemical compounds, including pesticides and insecticides, has caused a renewed interest in the quality of water. Many of these materials are known to be toxic and others have certain undesirable characteristics that interfere with the use of the water even when present in relatively small concentrations. In recent years instances of water pollution have been traced to sewage or wastewater sources containing synthetic detergents.
Substances that alter the quality of water as it moves over the surface of the earth may be classified under four major headings :
1. Physical : Physical characteristic are related to the quality of water for domestic use and are usually associated with the appearance of water, its color or turbidity, temperature, taste and odor.
2. Chemical : chemical differences between waters are sometimes evidenced by their observed reactions, such as the comparative performance of hard and soft waters in laundering.
3 . Biological : Biological agents are very important in their relation to public health and may also be significant in modifying the physical and chemical characteristics of water.
4. Radiological : Radiological factors must be considered in areas where there is a possibility that the water may have come in contact with radioactive substances.
(a) Physical Characteristic...
The water as used should be free from all impurities that are offensive to the sense o sight, taste, or smell. The physical characteristics of the water include turbidity, color, taste and odor, temperature, and foamability.
1. Turbidity : The presence of suspended material. Such as clay, silt, finely divided organic material, plankton, and other inorganic material in water is known as turbidity. Clay or other inert particles in drinking water may not adversely affect health, but water containing such particles may require treatment to make it suitable for its intended use. Following a rainfall, variation in the groundwater turbidity may be considered an indication of surface or other introduced pollution.
2. Color : Dissolved organic material from decaying vegetation and certain inorganic matter cause color in water. Occasionally, excessive blooms of algae or the growth of aquatic microorganisms may also impart color.
3. Taste and odour : Taste and odor in water can be caused by foreign matter, such as organic compounds, inorganic salts, or dissolved gases. Thses materials may come from domestic, agricultural or natural sources. Some substances found
naturally in groundwater, while not necessarily harmful, may impart a disagreeable taste or undesirable property to the water. Acceptable waters should be free from any objectionable taste or odor at point of use.
4. Temperature : The most desirable drinking waters are considerably cool and do not have temperature fluctuation of more than a few degreed. Groundwater and surface water from mountainous areas generally meet these criteria. The temperature of groundwater remains nearly constant throughout the year. Water from very shallow sources varies somewhat form one season to another, but water from deeper zones remains quite constant, its temperature being close to that for the average annual temperature at the surface.
5. Foamability : Since 1965, the detergent formulations have been changed to eliminate alkyl benzene sulfonate
(ABS), which was very slowly degraded by nature. The more rapidly biodegradable linear alkylate sulfonate (LAS) has been substituted in most detergents. Even LAS is not degraded very rapidly in the absence of oxygen a condition that exists
in cesspools and some septic tank file fields. Foam in water is usually caused by concentrations of detergents greater
than 1 mg/L. While foam itself is not hazardous, the user should understand that if enough detergent is reaching a water supply to cause a noticeable forth to appear on a galss of water, other possibly hazardous materials of sewage origin are also likely to be present.
(b) Chemical Characteristics...
The nature of the rocks that form the earths crust affects not only the quantity of water that may b recovered but also
its characteristics. As surface water seeps downward to the water table, it dissolves portions of the minerals contained
by soils and rocks. Groundwater, therefore, usually contains more dissolved minerals than surface water. The chemical characteristics of water in a particular locality can sometimes be predicted from analyses of adjacent water source.
These data are often available in published reports of the U.S.
Information that can be obtained from a chemical analysis :
The possible presence of harmful or disagreeable substances
The potential for the water to corrode parts of the water system
The tendency for the water to stain fixtures and clothing
1. Toxic substances : Water may contain toxic substances in solution. If analysis of the water supply shows that
these substances exceed the following concentration, the supply should not be used. Trihalomethanes may enter water from
industrial processes, but most commonly are formed during chlorination of water containing naturally occurring organics
such as humic acid. The trihalogenes are single-carbon organics with three of the carbon bonds being occupied by halogens such as chlorine, bromine or iodine. Chloroform is the most commonly occurring trihalomethanes are carcinogens, hence their presence in public water supplies is undesirable. Volatile organic chemicals (VOCs) are industrial chemicals, which have
been found to be widely distributed in both surface waters and groundwaters. Many of these substances are either known or suspended to be carcinogens.
2. Chlorides : Most waters contain some chloride in solution. The amount can be caused by the leaching of marine sedimentary deposits, by pollution from seawater, brine or industrial and domestic wastes. Chloride concentrations in
excess of about 250 mg/L usually produce a noticeable taste in drinking water. In areas where the chloride content is
higher than 250 mg/L and all other criteria are met, it may be necessary to use a water source that exceeds this limit.
An increase in chloride content in groundwater or surface water may indicate possible pollution from sewage sources, particularly if the normal chloride content is known to be low.
3. Copper : Copper is found in some natural waters, particularly in areas where these are deposits have been mined. Excessive amounts of copper can occur in corrosive water that passes through copper pipes. Copper in small amounts is not considered detrimental to health, but will impart an undesirable taste to the drinking water.
4. Fluorides : In some areas water sources contain natural fluorides. Fluoride, sometimes added to water as a means
of making teeth more resistant to decay, in higher concentration can cause permanent discoloration and loss of teeth and embrittlement of bones. The optimum fluoride level for a given area depends upon air temperature, since that is what primarily influences the amount of water people drink.
5. Iron : Small amounts of iron are frequently present in water because of the large amount of iron present in the soil and because corrosive water will pick up iron from pipes. The presence of iron in water is considered objectionable because it imparts a brownish color to laundered goods and affects the taste of beverages, such as tea and coffee. Recent studies indicate that eggs spoil faster when washed in water containing iron in excess of 10 mg/L. The recommended limit
for iron is 0.3 mg/L.
6. Lead : A brief or prolonged exposure of the body to lead can be seriously injurious to health. Prolonged exposure to relatively small quantities may result in serious illness or death. Excessive lead is corrosive water in contact with lead-painted roofs or the use of lead pipes. These conditions must be corrected to provide a safe water supply.
7. Manganese : There are two reasons for limiting the concentration of manganese in drinking water : (1) to prevent esthetic and economic damage (2) to avoid any possible physiological effects from excessive intake. The domestic user finds that manganese produced a brownish color in laundered goods and impairs thet taste of beverages, including coffee and tea.
8. Nitrates : Nitrate (NO3-) has caused methemoglobinemia in infants who have been given water or fed folrmulas prepared with water having high nitrates. A domestic water supply should not contain nitrate concentrations in excess of 45 mg/L. Nitrates in excess of normal concentrations, often in shallow wells, may be an indication of seepage from livestock manure deposits. In some polluted wells, nitrate will also be present in concentrations greater than 1 mg/L and is even more hazardous to infants. When the presence of high nitrate concentration is suspected, the water should not be used for infant feeding. Nitrate can interfere with oxygen transfer in the blood of infants, since it can be reduced to nitrite in immature digestive systems an, in that form, complex with hemoglobin.
9. Pesticides : Careless use of pesticides can contaminate water sources and make the water unsuitable for drinking.
Numerous cases have been reported where individual wells have been contaminated when the house was treated for termite control. The use of pesticides near well is not recommended. Chlorinated hydrocarbons are used as pesticides and
herbicides. These materials are relatively persistent both in nature and within the human body. Many have been shown to produce carcinogenic effects in laboratory animals.
10. Alkalinity : Alkalinity is imparted to water by bicarbonate, carbonate or hydroxide compounds. Knowledge of the
alkalinity sources is useful in the treatment of water supplies.
11. Hardness : Hard water and soft water are relatively terms. Hard water retards the cleaning action of soaps and detergents, causing an expense in the form of extra work and cleaning agents. Furthermore, when hard water is heated it
will deposit a hard scale with a consequent waste of fuel. The mineral content of groundwater reflects its movement through the minerals that make up the earths crust. Generally, groundwater in arid regions is harder and more mineralized than
water in regions of high annual rainfall. Also deeper aquifers are more likely to contain higher concentrations of minerals in solution, because the water has more time to dissolve the mineral rocks. Calcium and magnesium salts, which cause
hardness in water supplies, are divided into two general classifications: carbonate, or temporary hardness, and noncarbonate or permanent hardness. Carbonate or temporary hardness is so-called because heating the water will largely remove it. When the water is heated, bicarbonates break down into insoluble carbonates that precipitate as solid particles, which adhere to
a heated surface and the inside of pipes.
(c) Biological Characteristic...
Water for drinking and cooking purposes must be made free from disease producing organisms. These organisms include
bacteria, protozoa, viruses and helminthes (worms). Some organisms that cause disease in human originate with the fecal discharges of infected individuals. It is seldom practical to monitor and control the activities of human disease carriers. For this reason, it is necessary to exercise precautions against contamination of a normally safe water source or to institute treatment methods that will produce safe water.
The bacterial diseases include typhoid and cholera. Viral diseases associated with water include hepatitis. Certain fungi, notably Aspergillus are human pathogens while other is caused by a worm which may be transmitted through water via a snail carrier. Other infectious diseases are not generally transmitted by water. Organisms which cause infectious diseases are normally spread through the fecal and urinary discharges of sick persons and carriers, although there are some animal and soil reservoirs of protozoa and bacteria associated with gastroenteritis. Protection of water supplies against these agents is thus normally a matter of preventing discharges of inadequately treated wastewater into the source.
A great many microorganisms are found in water, most being of no health significance. It is difficult to test for the disease-causing species, whether viral, bacterial, fungal, or protozoal, since most grow rapidly only in their host. As
noted above these organisms are transmitted primarily through the feces and urine of infected persons. Water which shows evidence of such contamination is thus considered to be unfit for consumption. The possibility of such contamination is usually assessed by determining the number of coliform bacteria.
Contamination of water by biological agents can be minimized by :
1. Selecting water sources that do not normally support much plant or animal life
2. protecting the supply against subsequent contamination by biological agants
3. Minimizing entrance of fertilizing materials, such as organic and nutrient minerals
4. providing treatment for the destruction of biological life or its by-products
(d) Radiological Characteristics...
The development and use of atomic energy as a power source and mining of radioactive materials have made it necessary to establish limiting concentrations for the intake into the body of radioactive substances, including drinking water.
The effects of human exposure to radiation or radioactive materials are viewed as harmful and any unnecessary exposure
should be avoided. The concentrations of radioactive materials specified in the current Public Health Service Drinking
Water Standards are intended to limit the human intake of these substances so that the total radiation exposure of any individual will not exceed those. Water of high radioactivity is unusual. Nevertheless, it is known to exist in certain areas, either from natural or man-made sources.
Potable Reuse...
Potable reuse is the reinovation of sewage effluent to a water product suitable for human consumption and the recycling of that water into a supply system. Wastewater can, of course, also be reused for nonpotable purposes, and this is already
being done in industry, agriculture and recreation.
Potable reuse can be accomplished in any of three ways :
1. Direct potable reuse is the reintroduction of highly treated sewage effluent treated sewage effluent from the treatment plant directly back into the existing water distribution system. This is the classic pipe-to-pipe definition of reuse.
2. Planned indirect reuse involves the purposeful discharge of highly treated wastewater upstream from a water supply
intake.
3. Groundwater recharge involves either the injection of effluent into an aquifer that is the source of potable supply or
the spreading of effluent on the ground to allow it to filter down to the aquifer.
In each of these methods, renovated wastewater eventually reaches the home water tap. The environmental Protection Agency (EPA) has established a maximum contaminant level (MCL) for all these contaminants.
Water Demand...
The probable demand of water by a community is important because it fixes the sizes and capacity of water supply units.
The total quantity of water can be estimated by ascertaining different purposes for which the supply is necessary and the quantity likely to be used under each item of supply should be considered. The requirement is generally expressed in terms
of average number of litres of water per capita per day throughout the year. The various purposes for which the water is
used are given as below :
( 1 ) Domestic use : There is a wide variation in consumption of water for domestic uses depending upon climate conditions, customs and habits of consumers, pressure in distribution systems. For different types of buildings other
than residences, the water consumption varied. The water consumption by the livestock (animals) must be known specially
for farms and livestock areas.
( 2 ) Industrial use : The presence of industries in a town has a great effect upon total consumption. There is no direct relation of this consumption with the population and hence the actual requirements for all industries should be estimated. Large water-using industries such as canneries, chemical plants and refineries usually have their own supply and are not dependent on public agencies. Other industries such as those involved in high technology which have more modest process water requirements, may depend wholly on municipal supplies.
( 3 ) Irrigation : The demand for irrigation depends on type of the crop.
( 4 ) Fire demand : The demand of water for extinguishing fire is very small in a year but the rate of consumption
is large. The scheme should provide the necessary peak demand for fire fighting. The water requirement for extinguishing
fire depends on bulk, congestion and fire resistance of buildings. Indirectly we can say that it mainly depends on population. The minimum limit of fire demand is the amount and rate of supply that are required to extinguish the largest probable fire that may occur in a town. The following empirical formulae may be used where Q is the demand in litres per minute and P the population in thousands.
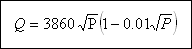
( 5 ) Recreational facilities : Recreational facilities such as swimming pools, bowling alleys, camps, resorts, and country clubs perform a wide range of functions involving water use.
Estimating Water Demand...
Water records of various types are kept by water supply agencies. These records usually include information on the amount
of water produced or withdrawn and discharged to the water supply system and the amount of water actually used (consumed). The distinction is important because more water is produced than is actually used by the consumer. The difference between these two values is the amount of water lost or unaccounted for in the distribution system plus the amount used for various public services that may be unmeterd. Therefore, in using water supply records to estimate wastewater flowrates, it is necessary to determine the amount of water actually used by the customers. Unaccounted water and losses do not reach the wastewater system and have to be excluded in making flow estimates. Data from municipal water-use records are analyzed in problem 1 to determine consumption and unaccounted system losses.
Problem 1...
A small community water supply agency furnishes water to 147 customers from a well supply. Water records are kept showing
the amount of water pumped to the system. The agency recently installed meters for all customers and total water sales are also kept. The following data were obtained :

From the water supply data, determine the amount of water consumed (gal/capita.d) and the amount of water that is
unaccounted system loss (as a percent of production). The average household size as determined by the local planning
agency is 2.43 persons per service.
Maximum Daily Demand : The average usage of water for domestic, commercial & industrial, public services such as
fire fighting and public buildings and unaccounted pipeline system losses and leakage are 220, 260, 30 and 90 liters per capita per day (L/c.d), respectively. The maximum daily water use ranges from about 120 to 400 percent of the average
daily use with a mean of about 180 percent.
Problem 2...
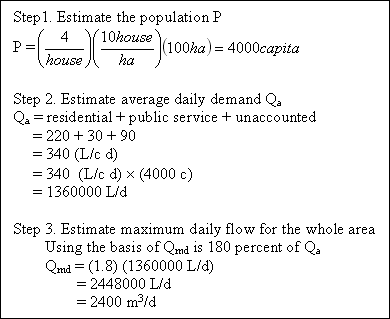
Assuming a high-value residential area of 100 hectares has a housing density of 10 houses per hectare with 4 persons per household, determine the maximum daily water demand.
Solution...
Fire Fighting Demand : Fire demand of water is often the determining factor in the design of mains. Distribution is
a sort-term, small quantity but with a large flow rate. The National Board of Fire recommended the following fire flow rate and population relationship :

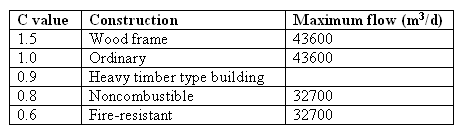
Table 5. Coefficient related to the type of construction...
Problem 3...
Calculate the water fire requirement for a 4-story building of heavy timber type building of 715 m2 of ground area.
Solution...

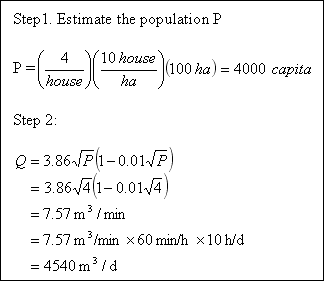
Problem 4...
Assuming a residential area of 100 hectares has a housing density of 10 houses per hectare with 4 persons per household. Estimate the water demand for fire fighting.
Solution...
Variation in demand from average : The water consumption varies throughout the year. In certain months, the demand
is maximum while in other months it is less. Similarly, in months of peak demand, there are peak days, i.e. on certain
days the consumption will be more (e.g. on holidays). So also in the day of peak demand, there are certain peak hours.
Thus the total water supply should be adequate for these peak hours. The maximum daily consumption is about 180 % of the average daily consumption. Now considering the peak hourly demand, it is seen that the demand is maximum during 6-8 am and then falls steadily till a minimum is reached at about 1.00 pm and again increases.