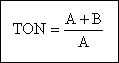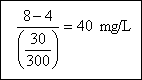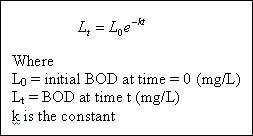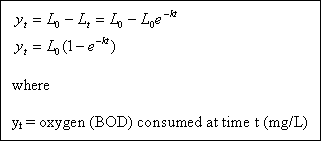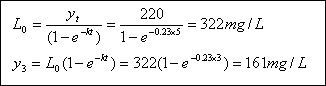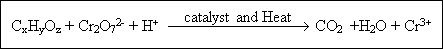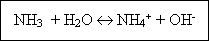Wastewater...
Wastewater is the combination of liquid and water-transported wastes from homes, commercial buildings, industrial
facilities, and institutions, along with any groundwater infiltration and surface water and stormwater inflow that may
enter the sewer system.
Sources of Wastewater...
1. Domestic Wastewater...
Domestic wastewater is the spent water originating from all aspects of human sanitary water usage. It typically constitutes a combination of flows from the kitchen, bathroom and laundry, toilets, baths, kitchen sinks, garbage grinders, dishwashers, washing machines and water softeners. Domestic wastewater, as the name implies, principally originates in residences and is also referred to as sanitary sewage. As such, commercial, institutional and industrial establishments contribute a domestic wastewater component to the sewer system resulting from human sanitary activity.
2. Industrial Wastewater...
Industrial wastewater emanates from the myriad of industrial processes that utilize water for a variety of purposes. This water is usually altered considerably in the process and may contain contaminants that degrade the water quality such as nutrients, suspended sediments, bacteria, oxygen demanding matter, and perhaps toxic substances.
Characteristics of Wastewater...
Units of Measurement for Physical and Chemical Parameters...
The results of the analysis of wastewater samples are expressed in terms of physical and chemical units of measurement.
(a) Physical and Chemical Characteristic...
The concentration of physical and chemical pollutants in domestic wastewater is quite variable and particularly dependent upon local conditions. In addition, the concentration of individual constituents is normally expected to vary with the time of the day, the day of the week and the month of the year. This variation results from the normal cycle of human activity during a given day, the difference between weekly and weekend domestic water usage, and fluctuations with the season of the year.
( 1 ) Odor...
Odors in domestic wastewater usually are caused by gases produced by the decomposition of organic matter or by substances added to the wastewater. Fresh, aerobic, domestic wastewater has been said to have the odor of kerosene or freshly turned earth, which is less objectionable than the odor of wastewater that has undergone anaerobic decompostion. Aged, septic
sewage is considerably more offensive. The characteristic rotten-egg odor of hydrogen sulfide and the mercaptants is indicative of septic sewage, which is produced by anaerobic microorganisms that reduce sulfate to sulfide.
Industrial wastewater may contain either odorous compounds or compounds that produce odors during the process of wastewater treatment.
The importance of odors at low concentrations in human terms is related to the psychological stress they produce rather than to the harm they do to the body. Offensive odors can cause poor appetite for food, lowered water consumption, impaired respiration, nausea and vomiting, and mental perturbation.
The threshold odor of a water or wastewater sample is determined by diluting the sample with odor-free water. The threshold odor number (TON) corresponds to the greatest dilution of the sample with odor-free water at which an odor is just perceptible. The recommended sample size is 200 mL. The numerical value of the TON is determined as follows :
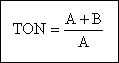
where A = mL of sample and B= mL of odor free water. The odor emanating from the liquid sample is determined with human subjects (often a panel of subjects) as discussed above.
( 2 ) Color...
Fresh sewage is typically gray in color. However, as the travel time in the collection system increases, and more anaerobic conditions develop, the color of the wastewater changes sequentially from gray to dark gray and ultimately to black.
( 3 ) Temperature...
Wastewater temperatures normally range between 10 and 20OC. In general, the temperature of the wastewater will be higher than that of the water supply. This is because of the addition of warm water from households and heating within the plumbing system of the structure. Depending on the geographic location, the mean annual temperature of wastewater varies.
The temperature of water is a very important parameter because of its effect on chemical reactions and reaction rates, aquatic life, and the suitability of the water for beneficial uses. Increased temperature, for example, can cause a change
in the species of fish that can exist in the receiving water body.
In addition, oxygen is less soluble in warm water than in cold water. The increase in the rate of biochemical reactions that accompanies an increase in temperature, combined with the decrease in the quantity of oxygen present in surface waters, can often cause serious depletion in dissolved oxygen concentrations in the summer months.
Optimum temperatures for bacterial activity are in the ranges from about 25 to 35OC. Aerobic digestion and nitrification stop when the temperature rises to 50OC. When the temperature drops to about 15OC, methane-producing bacteria become quite inactive, and at about 5OC, the autotrophic-nitrifying bacteria practically cease functioning.
( 4 ) Solids...
Analytically, the total solids content of a wastewater is defined as all matter that remains as residue upon evaporation at 103 to 105OC. Matter that has a significant vapor pressure at this temperature is lost during evaporation and is not defined as a solid. Settleable solids are those solids that will settle to the bottom of a cone-shaped container, called an Imhoff cone.
Total solids can be further classified as suspended (nonfilterable) or dissolved (filterable), by passing a known volume of sample through a glass-fiber filter (Whatman GF/C). If suspended solids are to be determined, the filter is dried and preweighed, a measured volume of sample is drawn through it, and it is dried and reweighed. The increase in weight divided
by the volume of the sample yields the concentration of suspended solids. A measured volume of the filtrate may be
evaporated to dryness and the residue weighed to determine the dissolved solids mass. This, divided by the sample volume, will give the dissolved solids concentration.
Each of the categories of solids may be further classified on the basis of their volatility at 550 +/- 50OC. The organic fraction will oxidize and will be driven off as gas at this temperature, and the inorganic fraction remains behind
as ash. Thus the terms volatile suspended solids and fixed suspended solids refer, respectively, to the organic and
inorganic (or mineral) content of the suspended solids.
Problem 1 : Analysis of Solids Data...
The following test results were obtained for a wastewater sample taken at the headworks to a wastewater treatment plant.
All of the tests were performed using a sample size of 50 mL. Determine the concentration of total solids, total volatile solids, suspended solids, and volatile suspended solids. The samples used in the solids analyses were all either evaporated, dried, or ignited to constant weight.
- Tare mass of evaporating dish = 53.5433 g
- Mass of evaporating dish plus residue after evaporation at 105OC = 53.5793 g
- Mass of evaporating dish plus rsidue after ignition at 550OC = 53.5772 g
- Tare mass of Whatman GF/C filter = 1.5433 g
- Residue on Whatman GF/C filter after drying at 105OC = 1.5553 g
- Residue on Whatman GF/C filter after ignition at 105OC = 1.5531 g
( 5 ) Density...
The density of wastewater row is defined as its mass per unit volume expressed as kg / m3. Density is an important physical characteristic of wastewater because of the potential for the formation of density currents in sedimentation tanks and in other treatment units. Density of wastewater are temperature dependent and will vary with the concentration of total solids in the wastewater.
( 6 ) Turbidity...
Turbidity, a measure of the light-transmitting properties of water, is another test used to indicate the quality of waste discharges and natural waters with respect to colloidal and residual suspended matter. The measurement of turbidity is based on comparison of the intensity of light scattered by a sample as compared to the light scattered by a reference suspension under the same conditions.
( 7 ) Chemical Compounds...
The discussion of chemic characteristics of wastewater is presented in four parts :
(i) Organic matter
(ii) Inorganic matter
(iii) Gases
(i) Organic matter : In a wastewater of medium strength, about 75 percent of the suspended solids and 40 percent of the filterable solids are organic in nature. These solids are derived from both the animal and plant kingdoms and the activities of man as related to the synthesis of organic compounds. Organic compounds are normally composed of a combination of carbon, hydrogen, and oxygen, together with nitrogen in some cases. The principal groups of organic substances found in wastewater are proteins (40 to 60 percent), carbohydrates (25 to 50 percent), and fats and oils (10 percent). Urea, the
chief constituent of urine, is another important organic compound contributing to wastewater. Because it decomposes so rapidly, undecomposed urea is seldom found in other than very fresh wastewater. Along with the proteins, carbohydrates,
fats and oils, and urea, wastewater contains small quantities of a large number of different synthetic organic molecules ranging from simple to extremely complex in structure. Typical examples, discussed in this section, include surfactants, organic priority pollutants, volatile pollutants compounds, and agricultural pesticides. Further, the number of such compounds is growing yearly as more and more organic molecules are being synthesized.
( a ) Carbohydrates : Widely distributed in nature, carbohydrates include sugars, starches, cellulose and wood fiber. All
are found in wastewater. Some carbohydrates, notably sugars are soluble in water; others, such as the starches, are insoluble.
( b ) Fats, oils and grease : Fats and oils are the third major component of foodstuffs. Fats and oils are contributed to domestic wastewater in butter, lard, margarine and vegetable fats and oils. Fats are also found in meats, seeds, nuts and
in certain fruits. The term grease as commonly used, includes the fats, oils, waxes and other related constituents found
in wastewater. The grease content of wastewater can cause many problems in both sewers and waste treatment plants, If grease is not removed before discharge of the waste, it can interfere with the biological life in the surface waters and create unsightly floating matter and films.
( c ) Surfactants : Surfactants, or surface-active agents, present in synthetic detergents, are large organic molecules
that are slightly soluble in water and cause foaming in wastewater treatment plants and in the surface waters into which
the waste effluent is discharged. During aeration of wastewater, these compounds collect on the surface of the air bubbles and thus create very stable foam.
Measurement of Organic Content...
Laboratory methods commonly used today to measure gross amounts of organic matter in wastewater include : (1) Biochemical oxygen demand (BOD); (2) Chemical oxygen demand (COD) (3) Total organic carbon (TOC).
( 1 ) Biochemical Oxygen Demand (BOD) : BOD is not a specific pollutant. BOD is a measure of the amount of oxygen required by bacteria and other microorganisms to oxidize any organic matter in the water biochemically. So BOD is an
indirect measure of the concentration of organic contamination in the water. The more organic matter present, the greater
the amount of oxygen that microorganisms will consume in oxidizing the wastes to CO2 and H2O. This process is called waste stabilization. BOD analysis dose not oxidize all of the organic matter present in the waste. Only
the organics that are biochemically degradable during the standard 5 day time are oxidized. A very low mount of BOD would indicate either clean water, or that the available microorganisms are uninterested in consuming the available organic compounds, or that the microorganisms are dead or dying.
Temperature has a pronounced effect on oxygen uptake, because metabolic activity increases significantly at higher temperature. The time allotted for the test is also important, since the amount of oxygen used increases with time.
Light is also an important variable since most natural waters contain algae and oxygen that can be replenished in the
bottle if light is available.
The BOD test has been standardized to be run in the dark at incubator at 20 OC for 5 days. The 5-day BOD, or
BOD5 is the oxygen used by microorganisms in the water sample during the first 5 days after sampling. Scientifically, 5 days is a compromise between running the test long enough to get reproducible results and running
it so long that anaerobic material and mold in the BOD bottles interferes. The BOD test is usually carried out in standard BOD bottle, about 300 ml volume. The test is begun by measuring the initial DO and final DO after 5 days.
For example sample A had an initial DO of 8 mg/L and in 5 days this drops to 2 mg/L.
The BOD therefore is : BOD5 = 8 2 = 6 mg/L
Sample B also has an initial DO of 8 mg/L, but the oxygen is used so fast that it drops to zero by the second day. Since there is no measurable DO left after 5 days, the BOD of sample B must be more than 8 0 = 8 mg/L, but we do not know how much more since the organisms in the sample might have used more DO if it had been available. Samples like this require repeating the 5-day BOD test after diluting the sample.
To ensure that meaningful results are obtained, the sample must be suitably diluted with a specially prepared dilution
water so that adequate nutrients and oxygen will be available during the incubation period. Normally, several dilutions
are prepared to cover the complete range of possible values.
When the sample contains a large population of microorganisms (untreated wastewter, for example), seeding is not
necessary. If required, the dilution water is seeded with a bacterial culture that has been acclimated to the organic matter or other materials that may be present in the wastewater. The seed culture that is used to prepare the dilution
water for the BOD test is a mixed culture. Such cultures contain large numbers of saprophytic bacteria and other organisms that oxidize the organic matter. In addition, they contain certain autotrophic bacteria that oxidize noncarbonaceous
matter. A variety of commercial seed preparations are also available.
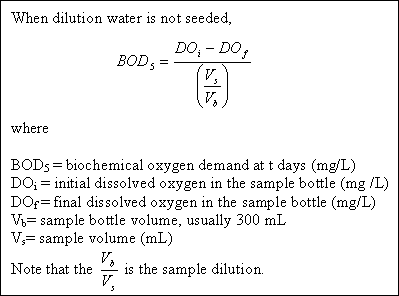
Problem 2 : BOD calculation...
Suppose that wastewater sample C had an initial DO of 8 mg/L and after 5 days this drops to 2 mg/L, is diluted by 30 : 300. The BOD of sample is therefore :
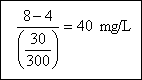
If we look more closely at the microbial process during the BOD test, we will find that the consumption of the organic matter, which we have measured as a change in dissolved oxygen level, occurs as a function several parameters, including
the number and types of microorganisms present, the temperature, the amount of dissolved oxygen available, and others. The rate of removal of BOD (the rate of oxidation of organic matter) can be approximated as a function of BOD (organic matter) remaining, or :
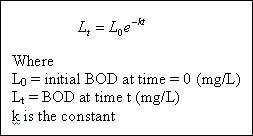
However we are more interested in the amount of oxygen consumed in some time period t, rather than the BOD remaining.
The oxygen consumed is the difference between the ultimate or total BOD ( L0 ), and the remaining BOD
( Lt )
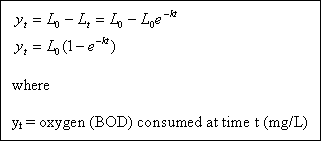
Following example demonstrates the use of these equations.
Problem 3 : BOD Calculation...
A typical wastewater has a BOD5 of approximately 220 mg/L. If the k for it is 0.23 day-1 (base e),
what is the ultimate BOD ? What is the 3 day BOD ?
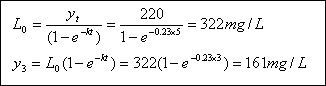
( 2 ) Chemical Oxygen Demand (COD) : The equivalent amount of oxygen required to oxidize any organic matter in a
water sample by means of a strong chemical oxidizing agent is called chemical oxygen demand. Because nearly all organic compounds are oxidized in the COD test and only some are decomposed during the BOD test, COD values are always higher than BOD values. One example of this is wood pulping waste, in which compounds such as cellulose are easily oxidized chemically (high COD) but are very slow to decompose biologically (low BOD).
Potassium dichromate (K2Cr2O7) is generally used as an oxidizing agent, in a moderately concentrated sulfuric acid (H2SO4). These were added to a measured amount of sample, a catalyst
(silver sulfate) is added to assist in the reaction and a complexing agent, mercuric sulfate. This mixture is boiled at
105OC for 2 hours, a process termed chemical digestion. This oxidize practically all of the organic carbon to
CO2 and H2O. However, as in the BOD analysis ammonia is not oxidized. In this reaction, the
oxidizing agent is hexavalent chromium, which is itself reduced to trivalent chromium. The reaction, which includes excess oxygen is :
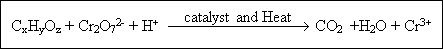
Where CxHyOz is ageneral expression for the organic compounds in question. The carbonate equilibrium results in some carbonate escaping from the reaction mixture as CO2. After boiling the excess
Cr+6 remaining is titrated against a reducing agent, usually ferrous ammonium sulfate. The difference between
the Cr+6 initially added as dichromate and the Cr+6 remaining is the chromate used to oxidize the organic compounds. COD is then proportional to the chromate Cr+6 used. To determine the amount of COD present
in the sample, measure the Cr+6 remaining after the digestion and compare this amount to the initial amount of Cr+6. The difference between the two is the COD of the sample.
The COD analysis is relatively fast compared with the BOD analysis (3 hours versus 5 days), and it is relatively
repeatable. Wastewater treatment personnel frequently use COD analysis to obtain information quickly for plant operation,
but BOD analysis is used for EPA required monitoring. The COD of a waste is, in general, higher than the BOD because more compounds can be chemically oxidized than can be biologically oxidized.
( 3 ) Total Organic Carbon : Another means for measuring the organic matter present in water is the TOC test, which
is especially applicable to small concentrations of organic matter. The test is performed by injecting a known quantity of sample into a high-temperature furnace or chemically oxidizing environment. The carbon dioxide that is produced is quantitatively measured by means of an infrared analyzer. Certain resistant organic compounds may not be oxidized, however, and the measured TOC value will be slightly less than the actual amount present in the sample.
(ii) Inorganic matter : The concentrations of inorganic substances in water are increased both by the geologic formation with which the water comes in contact and by the wastewaters, treated or untreated, that are discharged to it.
The natural waters dissolve some of the rocks and minerals with which they come in contact.
( a ) Nitrogen : Because nitrogen is an essential building block in the synthesis of protein, nitrogen data will be
required to evaluate the treatability of wastewater by biological processes. Insufficient nitrogen can necessitate the addition of nitrogen to make the waste treatable. Where control of algal growths in the receiving water is necessary to protect beneficial uses, removal or reduction of nitrogen in wastewaters prior to discharge may be desirable. Total nitrogen is a measure of the organic nitrogen, ammonia, nitrite and nitrate. Organic nitrogen is determined by the Kjeldahl method. The aqueous sample is first boiled to drive off the ammonia and then it is digested. During the digestion, the organic nitrogen is converted to ammonia. Total Kjeldahl nitrogen is determined in the same manner as organic nitrogen, except
that the ammonia is not driven off before the digestion step. Kjeldah nitrogen is, therefore, the total of the organic and ammonia nitrogen.
Ammonia : Ammonia nitrogen exists in aqueous solution as either the ammonium ion or ammonia, depending on the pH of the solution, in accordance with the following reaction :
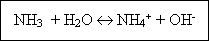
At pH levels above 7, the equilibrium is displaced to the left, at levels below pH 7, the ammonium ion is predominant. Ammonia is determined by raising the pH, distilling off the ammonia with the steam produced when the sample is boiled, and condensing the steam that absorbs the gaseous ammonia. The measurement is made colorimetrically, titrimetrically, or with specific-ion electrodes.
Nitrite : Nitrite nitrogen, determined colorimetrically, is relatively unstable and is easily oxidized to the nitrate form. It is an indicator of past pollution in the process of stabilization and seldom exceeds 1 mg/L in wastewater or 0.1 mg/L in surface waters or groundwaters. Nitrite present in wastewater effluents are oxidized by chlorine and thus increase the chlorine dosage requirements and the cost of disinfection.
Nitrate : Nitrate nitrogen is the most highly oxidized form of nitrogen found in wastewaters. Where secondary effluent is
to be reclaimed for groundwater recharge, the nitrate concentration is important.
The nitrogen present in fresh wastewater is primarily combined in proteinaceous matter and urea. Decomposition by bacteria readily changes the form to ammonia. The age of wastewater is indicated by the relative amount of ammonia that is present.
In an aerobic environment, bacteria can oxidize the ammonia nitrogen to nitrites and nitrates. The predominance of nitrate nitrogen in wastewater indicates that the waste has been stabilized with respect to oxygen demand. Nitrates, however, can be used by animals to form animal protein. Death and decomposition of the plant and nitrates can be reused to make protein by algae and other plants, it may be necessary to remove or to reduce the nitrogen that is present to prevent these growths.
Phosphorus : Phosphorous may appear in many forms in wastewater. Among the forms found are the orthophosphates, polyphophates, and organic phosphate. For our purpose, we will call of these together under the heading total
phosphorous (as P). Phosphorous is also essential to the growth of algae and other biological organisms. Because of
noxious algal blooms that occur in surface waters, these is presently much interest in controlling the amount of
phosphorous compounds that enter surface waters in domestic and industrial waste discharges and natural runoff.
Sulfur : The sulfate ion occurs naturally in most water supplies and is present in wastewater as well. Sulfur is required
in the synthesis of proteins and I released in their degradation. Sulfate is reduced biologically under anaerobic conditions to sulfide, which in turn can combine with hydrogen to form hydrogen sulfide (H2S). The following generalized reactions are typical.
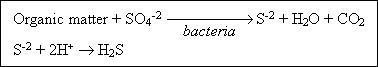
Hydrogen sulfide released to the atmosphere above the wastewater in sewers that are not flowing full tends to accumulate
at the crown of the pipe. The accumulated H2S can then be oxidized biologically to sulfuric acid, which is corrosive to sewer pipes. The H2S gas, which is evolved and mixed with the wastewater gas (CH4 + CO2), is corrosive to the gas piping, and, if burned in gas engines, the products of combustion can damage the engine and severely corrode exhaust gas heat recovery.
Heavy Metals : Trace quantities of many metals, such as nickel (Ni), manganese (Mn), lead (Pb), chromium (Cr), cadmium
(Cd), zinc (Zn), copper (Cu), iron (Fe), and mercury (Hg), are important constituents of most waters. Many of these metals are also classified as priority pollutants. Some of these metals are necessary for growth of biological life, and absence
of sufficient quantities of them could limit growth of algae, for example. The presence of any of these metals in excessive quantities will interfere with many beneficial uses of the water because of their toxicity; therefore, it is frequently desirable to measure and control the concentrations of these substances vary in complexity according to the interfering substances that may be present. In addition, quantities of many of these metals can be determined at very low concentrations by such instrumental methods as polarography and atomic absorption spectroscopy.
(iii) Gases : Gases commonly found in untreated wastewater include nitrogen (N2), oxygen (O2), carbon dioxide (CO2), hydrogen sulfide (H2S), ammonia (NH3) and methane (CH4). The first three are common gases of the atmosphere and will be found in all waters exposed to air. The latter three are derived from the decomposition of the organic matter present in wastewater. Although not found in untreated wastewater,
other gases with which the environmental engineer must be familiar include chorine (Cl2) and ozone
(O3) (disinfection and odor control), and the oxides of sulfur and nitrogen (combustion processes).
Methane : The principal by-product of the anaerobic decomposition of the organic mater in wastewater is methane gas.
Methane is a colorless, odorless, combustible hydrocarbon of high fuel value. Because methane is highly combustible and the explosion hazard is high, manholes and sewer junctions or junction chambers where there is an opportunity for gas to collect should be ventilated with a portable blower during and before the time required for employees to work in them. In treatment plants where methane is produced, employees should be instructed in safety measures to be maintained while working in and about the structures where gas may be present.
Hydrogen Sulfide : Hydrogen sulfide is formed, as mentioned previously, from the anaerobic decomposition of organic matter containing sulfur or from the reduction of mineral sulfides and sulfates. It is not formed in the presence of abundant
supply of oxygen. This gas is a colorless, inflammable compound with the characteristic odor of rotten eggs. The blackening of wastewater and sludge usually results from the formation of hydrogen sulfide that has combined with the iron present to form ferrous sulfide (FeS). Various other metallic sulfides are also formed. Although hydrogen sulfide is the most important gas formed from the standpoint of odors, other volatile compounds such as indol, skatol, and mercaptants, which may also be formed during anaerobic decomposition, may cause odors far more offensive that that of hydrogen sulfide.
( b ) Biological Constituents...
The environmental engineer must have considerable knowledge of the biological characteristics of wastewater. The engineer must know (1) the principal groups of microorganisms found in surface water and wastewater as well as those responsible for biological treatment, (2) the pathogenic organisms found in wastewater, (3) the organisms used as indicators of pollution
and their significance (4) the methods used to enumerate the indicator organisms and (5) the methods used to evaluate the toxicity of treated wastewaters.
The biological constituents found in domestic wastewater are extremely important due to the public health considerations involved, which stem from the source and nature of the microorganisms present. Many of the waste substances present in domestic wastewater are organic and serve as food for saprophytic microorganisms that live on dead organic matter. As a result, domestic wastewater is unstable, biodegradable and putrescible. In addition to the microorganisms that enter
domestic wastewater at its source, many also come from the soil by infiltration into the collection system. Thus, it is possible to find almost every type of microorganism in sewage. Approximately 105 bacteria per milliliter will be found in fresh domestic wastewater, whereas the bacteria count will rise to 107 to 108 bacteria per milliliter in stale sewage.
Of the microorganisms present in domestic wastewater, bacteria, protozoa, fungi, algae and viruses are of the greatest significance to the environmental engineer. Bacteria pay a fundamental role in the decomposition and stabilization of
organic matter both in nature and in wastewater treatment plants. Additionally, of the pathogenic organisms found in
domestic wastewater, bacteria are the most numerous and are responsible for diseases of the gastrointestinal tract, such as typhoid and paratyphoid fever, dysentery, diarrhea and cholera. These pathogenic microorganisms are excreted by human beings who are infected with or are carriers of a particular disease. Since the identification of pathogens in wastewater is extremely difficult and time consuming, coliform bacteria are utilized in the analysis of wastewater for the presence of human wastes and hence pathogenic organisms.
Protozoa feed on bacteria and are essential in the operation of biological treatment processes and in the purification of streams, since they maintain a natural balance among the different groups of microorganisms. Fungi are molds that have the ability to survive at low pH values, making them significant in biologically treating some industrial wastes and composting solid organic wastes. Algae are significant because of their ability to reproduce rapidly under the proper conditions and create algal blooms in surface waters. In addition to the nuisance associated with such bloom, algae also create taste and odor problems when present in water, thus diminishing its value for water supply purposes.
Pathogenic Organisms...
Pathogenic organisms found in wastewater may be discharged by human beings who are infected with disease or who are carriers of a particular disease. The usual bacterial pathogenic organisms that may be excreted by man cause diseases of the gastrointestinal tract such as typhoid and paratyphoid fever, dysentery, diarrhea and cholera. Because these organisms are highly infectious, they are responsible for many thousands of deaths each year in areas with poor sanitation, especially in the tropics.
Use of Indicator Organisms...
Because the number of pathogenic organisms present in wastes and polluted waters are few and difficult to isolate and identify, the coliform organism. The intestinal tract of man contains countless rod-shaped bacteria known as coliform organisms. Each person discharges from 100 to 400 billion coliform organisms per day, in adition to other kinds of bacteria. Thus, the presence of coliform organisms is taken as an indication that pathogenic organisms may also be present, and the absence of coliform organisms is taken as an indication that the water is free from disease producing organisms. The
coliform bacteria include the genera Escherichia and Aerobacter. The use of coliform as indicator organisms is complicated
by the fact that aerobacter and certain Escherichia can grow in soil. Thus, the presence of coliforms does not always mean contamination with human wastes. Apparently, Escherichia coli (E.coli) are entirely of fecal origin. There is difficulty in determining E.coli to the exclusion of the soil coliforms; as a result, the entire coliform group is used as an indicator
of fecal pollution. In recent years, tests have been developed that distinguish among total coliforms, fecal coliforms, and fecal streptococci; and all three are being reported in the literature.
Enumeration of Coliform Organisms...
The standard test for the coliform group may be carried out using either the mutipletube fermentation technique or by the membrane filter technique. The complete multiple-tube fermentation procedure for total coliform involves three test phases, identified as the presumptive, confirmed and completed test. A similar procedure is available for the fecal coliform group
as well as for other bacterial groups. The presumptive test is based on the ability of the coliform group to ferment lactose broth, producing gas. The confirmed test consists of growing cultures of coliform bacteria from the presumptive test on a medium that suppresses the growth of other organisms. The completed tests is based on the ability for the cultures grown in the confirmed test to again ferment the lactose broth. For most routine wastewater anlyses, only the presumptive test is performed.
Multiple-Tube Fermentation Technique : The multiple tube fermentation technique is based on the principle of dilution to extinction. The test may be described as follows. The next step is to transfer a 1-ml sample from each of the serial dilutions to each of five fermentation tubes containing a suitable lactose culture medium and an inverted gas collection tube. For total coliform the inoculated tubes are incubated in a water bath at 35 + / - 0.5OC for 24 + / - 2 hr. The accumulation of gas in the inverted gas collection tubes after 24 hr is considered to be a positive reaction. The results for each dilution are reported as a fraction, with the number of positive tubes over the total number of tubes. For example, the fraction 3 / 5 denotes three positive tubes in a five-tube sample. The test for fecal coliform is similar with the exception that a different culture medium is used and the inoculated tubes are first incubated at 35 + / - 0.5OC for 3 hr and then incubated in a water bath at 44.4 + / - 0.2OC for 21 + / - 2 hr. Estimation ofbacteril numbers using the test results from the multiple-tube technique is considered subsequently.
Estimation of coliform Densities...
Concentrations of total coliform bacteria are most often reported as the most probable number per 100 mL (MPN/100 mL).
The MPN will be determined using three different techniques :
(a) Poisson equation
(b) Thomas equation
(c) The MPN table
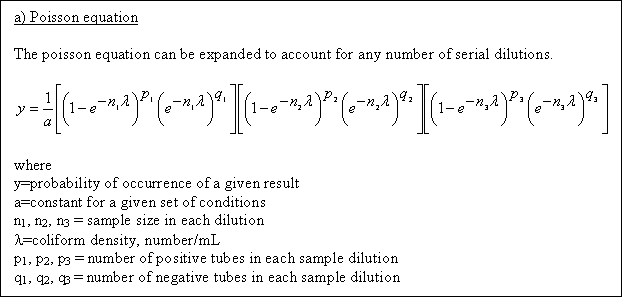

Comment : With the availability of powerful hand calculators, the use of the complete Poisson equation is no longer
a major undertaking. However, because of established practice, many analytical laboratories are continuing to use the MPN tables in Standard Methods as the basis for reporting MPN values.
MPN Tables...
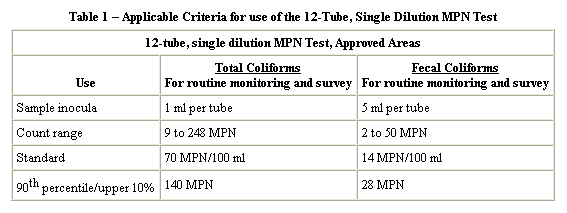
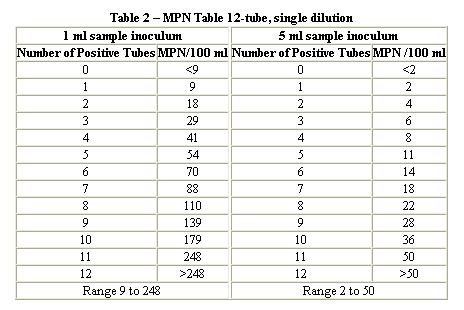
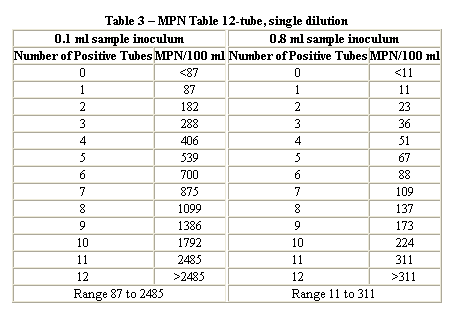
Problem 3 : Calculation of MPN...
A bacterial analysis for a surface water yielded the following results for the standard confirmed test for total coliform. Determine the coliform density (MPN) using three different methods.

Membrane Filter Technique...
The membrane filter technique can also be used to determine the number of coliform organisms that are present in water.
The determination is accomplished by passig a known volume of water sample through a membrane filter that has a very small pore size. The bacteria are then contacted with an agar that contains nutrients necessary for the growth of the bacteria. After incubation, the coliform colonies can be counted and the concentration in the original water sample determined. The membrane-filter technique has the advantage of being faster than the MPN procedure and of giving a direct count of the
number of coliforms. Both methods are subject to limitations.