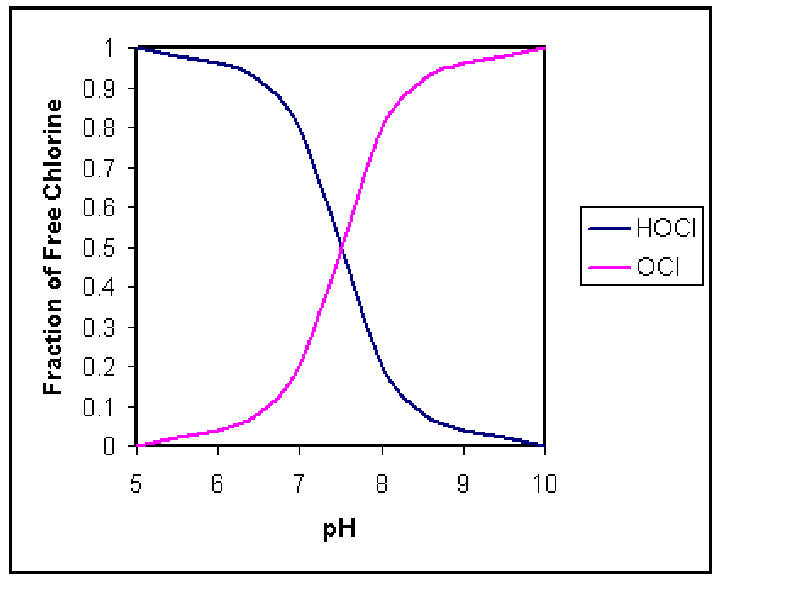
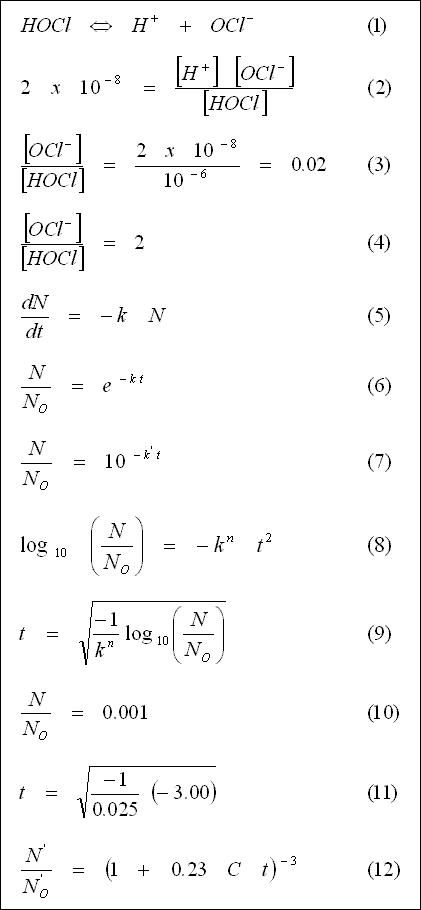Example 5-4 :
Typical settlement curves of sand of density 2,600 kg / m 3 ; anthracite of density 1,400 kg / m 3 and plastic of density 1,050 kg / m 3 are given in figure shown below.
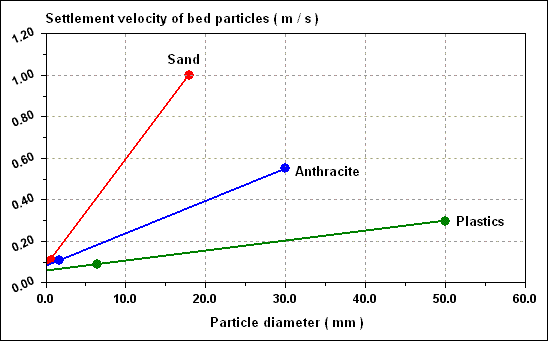
Calculation :
Available anthracite has a grain size between 1.4 mm and 1.7 mm, i.e. a range of settling velocities ( assuming spherical particles ) of 0.11 to 0.09 m / s when NU is 1.2 x 10 - 6 m 2 / s. Plastic particles less than 6.5 mm diameter would have a settlement velocity less than the smallest anthracite particles. In practice, plastic media of 2.0 to 3.0 mm diameter having settlement velocities of 0.033 to 0.040 m / s would be suitable. Sand of greater than 0.65 mm diameter would have velocities greater than the largest anthracite grains, so sand of perhaps 0.7 to 0.9 mm diameter, with settlement velocities of 0.12 to 0.15 m / s would be satisfactory to form a lower bed. The situation would then be as in table given below.