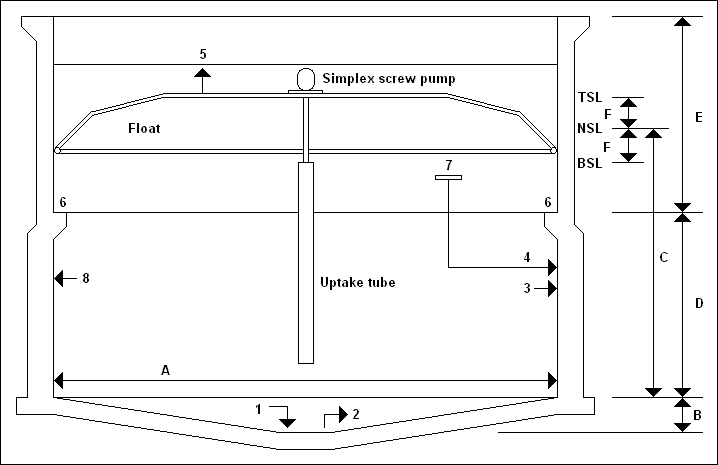
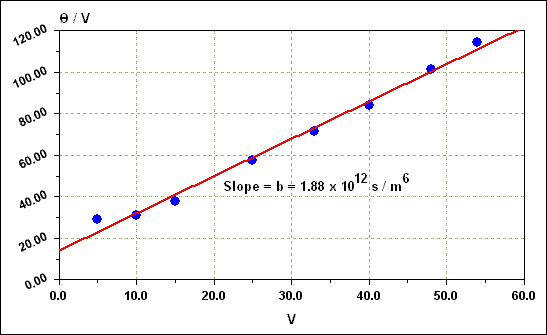
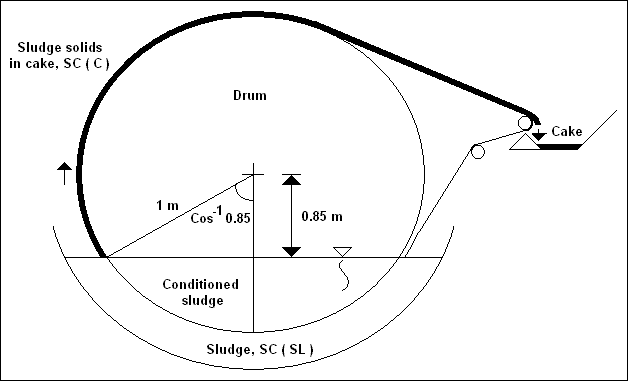
| Symbol |
Definition |
Units |
| MC |
Moisture content. Mass of liquid in sludge /
mass of sludge |
% |
| SC |
Solids content. Mass of solids ( dry mass )
in sludge / mass of sludge
|
% |
| V |
Volume of sludge or filtrate |
m 3 |
| M S |
Mass of dry solids in sludge |
kg |
| M W |
Mass of liquid in sludge |
kg |
| VM |
Volatile matter ( = organic content ) |
% |
| TS |
Total solids |
kg |
| X |
Volume of digester gas used in boiler |
m 3 |
| r |
Specific resistance to dewaterability |
m / kg |
| C |
Solids concentrations in sludge |
kg / m 3 |
| THETA |
Filtration time |
s |
| MU |
Absolute viscosity of filtrate |
N . s / m 2 |
| P |
Pressure ( difference ) across filter |
N / m 2 |
| A |
Filter area |
m 2 |
| R M |
Resistance of filter paper, cloth or septum |
1 / m |
| b |
Slope of THETA / V versus V plot |
s / m 6 |
| r O |
Specific resistance constant |
- |
| S |
Coefficient of compressibility |
Dimensionless |
| C T |
Mass of solids / unit volume of liquid
in the sludge |
kg / m 3 |
| Y |
Yield of vacuum filter |
kg / m 2 . s |
| A F |
Area of cake formation as fraction of
total area |
- |
| THETAR |
Time of l revolution |
s |
| f E |
Cake correction factor, ratio mass of
liquid / unit mass sludge : mass of filtrate / unit mass sludge dewatered |
- |
| SC ( Sl ) |
Solids content of wet sludge |
Fraction |
| SC ( C ) |
Solids content of sludge as it
emerges from the filter bath |
Fraction |
| T |
Time of pressure filtration |
h |
| k |
Constant of proportionality, emprical |
kg 2 / m 2 . s for
T in s |
| SC ( F ) |
Final cake solids content at end of
filter pressing |
Fraction |
| H |
Heat energy required to raise water to
incinerator exit temperature |
J |
| T L |
Inlet temperature of sludge |
O C |
| T E |
Exit temperature of exhaust gases |
O C |
| K |
Calorific value or energy / volume of
combustible material |
J / kg |
| CM |
Combustible material ( almost equivalent
to organic material ) |
- |
| Z |
Fraction of calorific value taken up by
incinerator heat loss |
- |