Chemical Treatment - 05...
Chapter 5 - Carbonate Precipitation...
5-1. Introduction...
Dissolved heavy metals can be removed from wastewaters by direct precipitation using a carbonate precipitating agent,
such as soda ash, Na 2 CO 3 , sodium bicarbonate, Na(HCO 3 ), or calcium carbonate,
CaCO 3 . Carbonate precipitation is an effective treatment alternative to hydroxide precipitation. The
solubilities of metal-carbonates depend on the specific metal ion precipitated and the pH of the wastewater. Generally,
the solubilities of metalcarbonates are intermediate between metal-hydroxide and metal-sulfide solubilities (see
Table 2-1).
( a ) Carbonate precipitation is oftentimes preferred over hydroxide precipitation for the removal of cadmium, lead, and
nickel. Industry prefers the precipitate cadmium carbonate to cadmium hydroxide for metals recovery processes. Also, lead
and nickel precipitation using calcium carbonate gives lower final residual metals concentrations than those of hydroxide.
( b ) Carbonate precipitation processes are not significant sources of air emissions. However, this technology may release
gaseous CO 2 if it is not operated correctly (EPA, 1987). In addition, the process produces metal-carbonates
and metal-hydroxides, and the resulting sludge is classified as a hazardous waste under 40 CFR 261 (Waste Code F006).
Therefore, sludges from carbonate precipitation processes may need to be encapsulated or fixed in some other way to stop
the metals from leaching in an acidic environment.
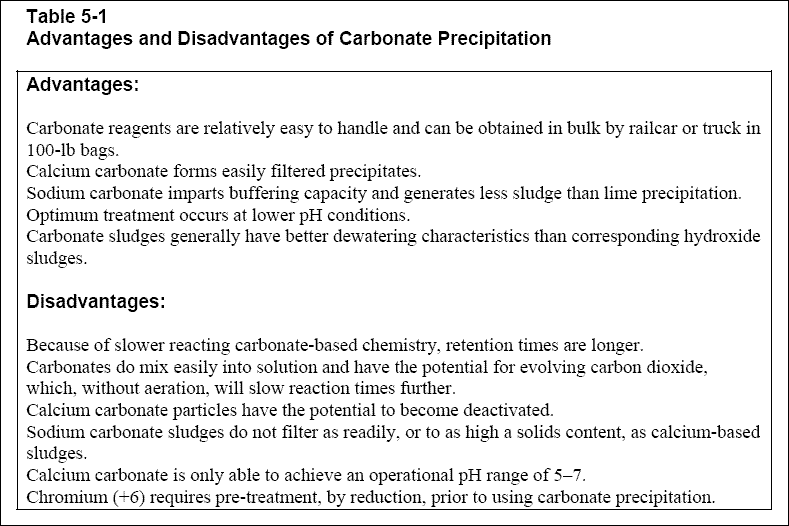
5-2. Advantages and Disadvantages of Carbonate Precipitation...
( a ) The main advantage of using carbonate precipitation is that it can operate at a lower pH range, typically between 7
and 9 (EPA, 1987). At this range, adjusting pH after precipitation wouldn’t normally be required. Also, carbonate
precipitation is competitively priced in relation to hydroxide precipitation, and carbonates form easily filterable
precipitates.
( b ) There are a few disadvantages of using carbonate precipitation. The treatment chemicals used for this process tend to
be abrasive and can damage feed equipment. In addition, the sludge produced in this process is gelatinous and difficult to
settle, and pre-treatment of chromium (+6) by reduction is required. A summary of the advantages and disadvantages of using
carbonate precipitation is shown in Table 5-1.
 5-3. Carbonate Precipitation Using Calcium Carbonate...
a. Limestone is available in either high calcium, CaCO 3 or dolomitic, CaCO 3 MgCO 3
form. Both types are available in powder or crushed stone form. Owing to its faster reaction rate and its more widespread
availability, the high calcium form is more often used. Powder form is desirable because both reactivity and completeness
of reaction increase proportionately to the available surface area (EPA, 1987).
5-3. Carbonate Precipitation Using Calcium Carbonate...
a. Limestone is available in either high calcium, CaCO 3 or dolomitic, CaCO 3 MgCO 3
form. Both types are available in powder or crushed stone form. Owing to its faster reaction rate and its more widespread
availability, the high calcium form is more often used. Powder form is desirable because both reactivity and completeness
of reaction increase proportionately to the available surface area (EPA, 1987).
( b ) The primary advantage of limestone is the low cost and widespread availability of the reagent. The main disadvantage
of calcium carbonate precipitation is that it is only effective for precipitating metal ions in its operational range (5.0
to 7.0) (EPA, 1987). Attempts have been made to use lime in combination with limestone as dual alkali. The limestone is
used as a pretreatment step to raise the pH to about 6.0, with lime completing the precipitation process. The
limestone/lime process is typically more complicated than a simple lime process; however, in high volume applications, the
savings in reagent may offset any increase in capital costs (EPA, 1987).
5-4. Carbonate Precipitation Using Sodium Carbonate...
Sodium carbonate, Na 2 CO 3 is marketed most commonly as an anhydrous powder. Soda ash is an
alternative to sodium hydroxide for acidic-metals waste streams, which lack buffering capacity. Through the use of
sodium carbonate (a weak base), a buffering capacity will be imparted, thereby allowing pH to be controlled and
precipitation to take place within the neutral range. Buffering agents produce a smaller change in pH per unit addition
than comparable unbuffered reagents, such as lime and caustic.
( a ) Precipitation, using soda ash, proceeds at much slower rates than comparable hydroxide methods, such as lime or
caustic. Accordingly, reactors should be sized to provide a minimum of 45 minutes of hydraulic retention time (EPA, 1987).
Because soda ash is commercially available only in dry form, on-site batch mixing and solution preparation facilities,
similar to those for hydrated lime, are required (EPA, 1987). Because of its solubility, chemical solution feed strength
of only 20% by weight can be maintained at ambient temperatures without salt recrystallization. To maintain homogeneity,
continuous mixing of the solution feed is recommended. Materials suitable for handling the compound, or its solutions,
include plastic, iron, rubber, and steel. Sodium carbonate is shipped in bags, barrels, or in bulk.
( b ) Soda ash provides the advantage of lower sludge generation rates (lower than using calcium carbonate) since
sodium-based end products are more soluble than calcium-based products. However, sodium-based sludges do not filter as
readily or attain as high a solids content as calcium-based sludges. In addition, the supernatant may not be as low in
metals content or total dissolved solids as when lime is used as the precipitating agent (EPA, 1987).


