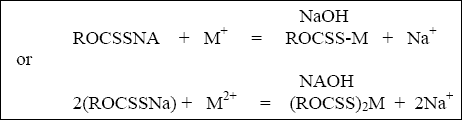Chemical Treatment - 06...
Chapter 6 - Other Precipitation Techniques...
6-1. Introduction...
Other precipitation techniques discussed below include xanthate precipitation and combined precipitation.
6-2. Xanthate Precipitation...
( a ) Heavy metals can also be removed from wastewaters by xanthate precipitation. Xanthate precipitation is a relatively
new technology compared to other precipitation methods. Xanthates are sulfonated organic compounds. The xanthate acts as
an ion exchange material, where heavy metals ions are replaced with sodium and magnesium. Starch xanthate (SX) treatment
has been demonstrated numerous times at full-scale, (EPA, 1989) and has proven ability to remove the following heavy
metals : Cd + 2 , Cr + 3 , Cu + 3 , Fe + 2 , Pb + 2 ,
Mn + 2 , Hg + 2 , Ni + 2 , Ag + , Zn + 2 (Anderson, 1994). For
mixed-metal solutions, the hierarchy for selective removal of some cations and heavy metals by xanthate precipitation is
in the following : Na << Ca-Mg-Mn < Zn < Ni < Cd < Pb < Cu-Hg. The xanthate-metal precipitation process can be represented
as follows :
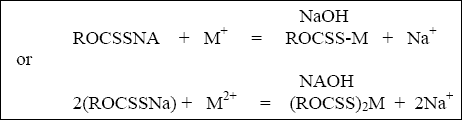
where M + and M + 2 are the metal ions and NAOH indicates that the reaction occurs at a high pH
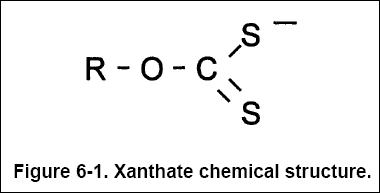
(pH typically greater than 9.0). ROCSS represents the xanthate material with a chemical structure shown in Figure 6-1,
where "R" denotes any organic compound.
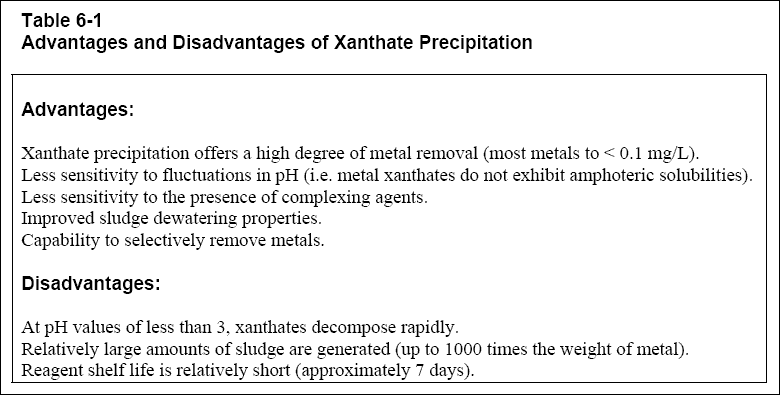
( b ) Xanthate precipitation offers several advantages and disadvantages as outlined in Table 6-1, below. Suggested
references for xanthate precipitation include Anderson (1994), and EPA (1989).
 6-3. Combined Precipitation...
With the exception of hydroxide precipitation, each precipitation method involves a combined precipitation system, because
precipitations are generally performed at a particular pH. For example, when employing sulfide precipitation at pH levels
greater than 6.0, hydroxide precipitation can also occur. Numerous bench scale treatability testing studies have been
conducted using combined precipitation. A suggested reference summarizing the results of these studies is Anderson (1994).
6-3. Combined Precipitation...
With the exception of hydroxide precipitation, each precipitation method involves a combined precipitation system, because
precipitations are generally performed at a particular pH. For example, when employing sulfide precipitation at pH levels
greater than 6.0, hydroxide precipitation can also occur. Numerous bench scale treatability testing studies have been
conducted using combined precipitation. A suggested reference summarizing the results of these studies is Anderson (1994).