Chemical Treatment - 07...
Chapter 7 - Coagulation and Flocculation...
7-1. Introduction...
Coagulation and flocculation are used to remove the insoluble and colloidal heavy metal precipitates formed during the
precipitation step. Colloidal heavy metal precipitates are tiny particles that possess electrical properties, which create
repelling forces and prevent agglomeration and settling. Coagulation is the process of making the particle less stable
by neutralizing its charge, thus encouraging initial aggregation of colloidal and finely divided suspended matter. Particles
no longer repel each other, and can be brought together.
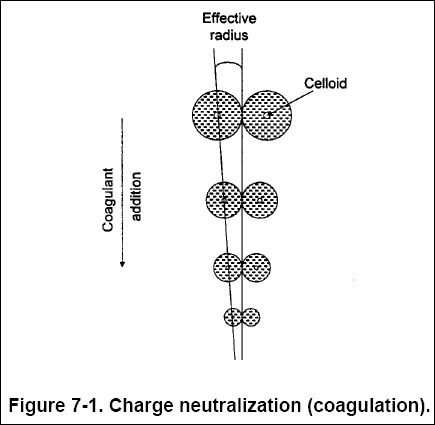
( a ) When suspended in water, the charge on organic and inorganic colloids is typically negative. Because of electrostatic
forces, the negative colloid charge attracts positive ions. Figure 7-1 illustrates how coagulants reduce the electric
charges on the colloidal surfaces, allowing colloidal particles to join.
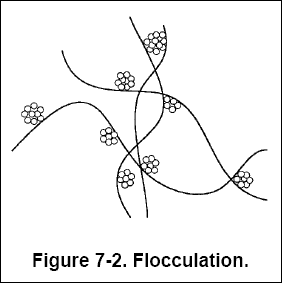
( b ) Flocculation is the process of bringing together the destabilized or "coagulated" particles to form a larger
agglomeration of floc by physical mixing or addition of chemical coagulant aids, or both. Figure 7-2 illustrates the
bridging of agglomerated colloidal particles to form settable flocs.
 7-2. Theory and Discussion...
Zeta potential is a measurable quantity and is sometimes used to predict the potential for coagulation. Effective
coagulation has been found experimentally to occur at zeta potential values ranging from ± 0.5 mV. More information on
zeta potential is presented in chapter 10. Inorganic compounds (typically iron and aluminum derivatives) are commonly
used as coagulants. During dissolution, the cations serve to neutralize the particle charge and the effective distance of
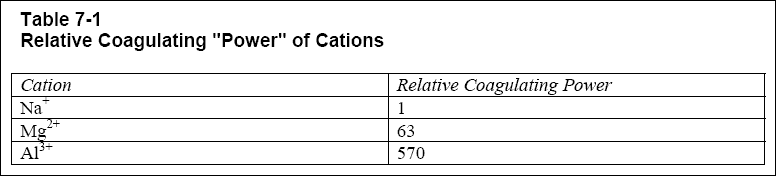
the double layer, thereby reducing the zeta potential. In inorganic coagulants, a trivalent ion can be as much as 1,000
times more effective than a monovalent ion. This is the reason that alum and iron salts are extremely efficient coagulants.
Table 7-1 illustrates the increasing coagulation "power" with cation reactivity.
7-2. Theory and Discussion...
Zeta potential is a measurable quantity and is sometimes used to predict the potential for coagulation. Effective
coagulation has been found experimentally to occur at zeta potential values ranging from ± 0.5 mV. More information on
zeta potential is presented in chapter 10. Inorganic compounds (typically iron and aluminum derivatives) are commonly
used as coagulants. During dissolution, the cations serve to neutralize the particle charge and the effective distance of
the double layer, thereby reducing the zeta potential. In inorganic coagulants, a trivalent ion can be as much as 1,000
times more effective than a monovalent ion. This is the reason that alum and iron salts are extremely efficient coagulants.
Table 7-1 illustrates the increasing coagulation "power" with cation reactivity.
( a ) Colloids can also be destabilized through the addition of polyelectrolytes, which can bring the system to the
isoelectric point without a change in pH.
( b ) These polyelectrolytes are 10 to 15 times more effective than alum as a coagulant; however, they are considerably
more expensive.
( c ) The coagulation and flocculation processes typically include the following four steps :
• If necessary, adding alkalinity (bicarbonate has the advantage of providing alkalinity without raising pH).
• Adding the coagulant and coagulant aid to the influent after precipitation.
• Rapid mixing of the coagulant throughout the liquid.
• Adding the coagulant aid, followed by slow and gentle mixing to allow for contact between small particles and subsequent
agglomeration into larger particles.
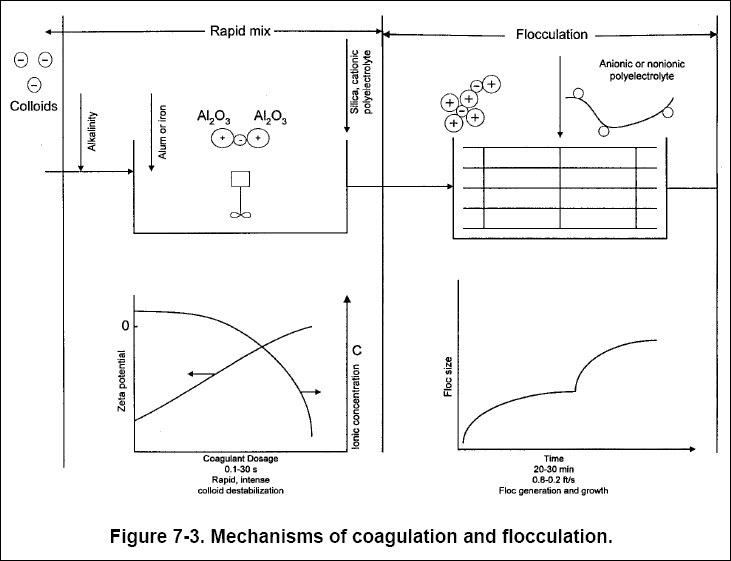
Coagulant aids typically require a short, rapid mix followed by gentle mixing (see Chapter 9). Figure 7-3 shows the
mechanisms of the coagulation and flocculation processes.
( d ) The overall success of the coagulation and flocculation processes depends on the flocculating and settling
characteristics of the particles. The frequency of collisions between the particles is directly proportional to the rate
at which coagulated particles coalesce. The collision frequency is proportional to the concentration of particles and the
difference in settling velocities. Because the total number of particle collisions increases with time, the degree of
flocculation generally increases with residence time. The agglomeration of particles cannot be predicted from collision
frequency alone. The rate of flocculation depends upon several factors, which include :
• The nature of the particle surface.
• The presence of charges.
• The shape of the particles.
• The density of the particles.





