Chemical Treatment - 08...
Chapter 8 - Coagulants, Polyelectrolytes, and Coagulant Aids...
8-1. Introduction...
Numerous chemicals are used in coagulation and flocculation processes. There are advantages and disadvantages associated
with each chemical. The designer should consider the following factors in selecting these chemicals :
• Effectiveness.
• Cost.
• Reliability of supply.
• Sludge considerations.
• Compatibility with other treatment processes.
• Environmental effects.
• Labor and equipment requirements for storage, feeding, and handling.
( a ) A suggested reference for summarizing the above factors is EPA (430/9-79-018), Chemical Aids Manual for Wastewater
Treatment Facilities. For a more complete bibliography, see Appendix A, References.
( b ) Coagulants and coagulant aids commonly used are generally classified as inorganic coagulants and polyelectrolytes.
Polyelectrolytes are further classified as either synthetic-organic polymers or natural-organic polymers.
8-2. Inorganic Coagulants...
The three main classifications of inorganic coagulants are :
• Aluminum derivatives.
• Iron derivatives.
• Lime.
With exception of sodium aluminate, all common iron and aluminum coagulants are acid salts and, therefore, their
addition lowers the pH of the treated water. Depending on the influent's pH and alkalinity (presence of
HC0 3 – , C0 3 – 2 , and OH – ), an alkali, such as lime or
caustic, may be required to counteract the pH depression of the coagulant. This is important because pH affects both
particle surface charge and floc precipitation during coagulation. The optimum pH levels for forming aluminum and iron
hydroxide flocs are those that minimize the hydroxide solubility (EPA, 1987). However, the optimum pH for coagulating
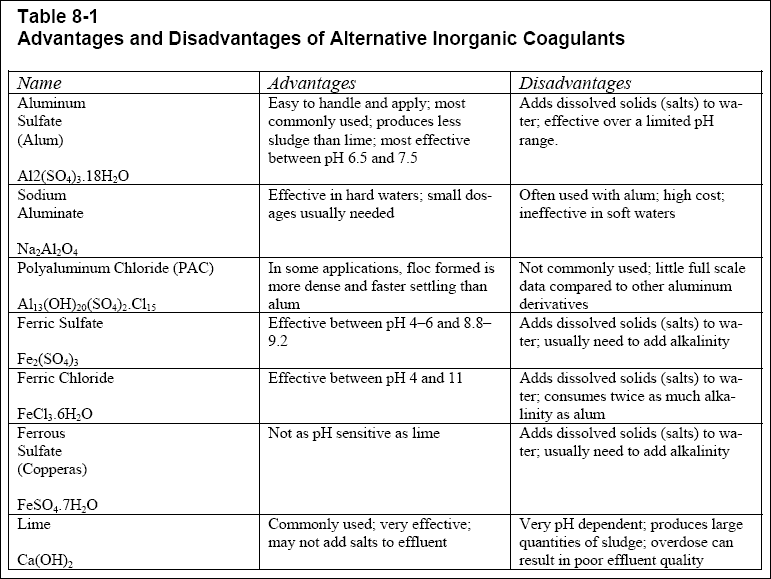
suspended solids does not always coincide with the optimum pH for minimum hydroxide floc solubility. Table 8-1 lists
several common inorganic coagulants along with associated advantages and disadvantages.
( a ) Aluminum Derivatives. Common aluminum coagulants include aluminum sulfate (alum), sodium aluminate, and polyaluminum
chloride. Dry alum is available in several grades, with a minimum aluminum content (expressed as %A1203) of 17%. Liquid
alum is about 49% solution, or approximately 8.3% by weight aluminum as A1203. Alum coagulation works best for a pH range
of 5.5 to 8.0; however, actual removal efficiency depends on competing ions and chelating agent concentrations.
( 1 ) Sodium aluminate is an alternative to alum and is available in either dry or liquid forms, containing an excess of
base. Sodium aluminate provides a strong alkaline source of water-soluble aluminum, which is useful when adding sulfate
ions is undesirable. It is sometimes used in conjunction with alum for controlling pH.
( 2 ) Polyaluminum chloride (PAC), another aluminum derivative, is a partially hydrolyzed aluminum chloride solution.
Although still not widely used, it has been reported to provide stronger, faster settling flocs than alum in some
applications.
( b ) Iron Derivatives. Iron coagulants include ferric sulfate, ferric chloride, and ferrous sulfate (copperas). Compared
to aluminum derivatives, iron coagulants can be used successfully over a much broader pH range of 5.0 to 11.0. However,
when ferrous compounds are used, the solution is typically chlorinated before it is sent into the coagulation vessel. As
this reaction produces both ferric chloride and ferric sulfate, chlorinated ferrous sulfate has the same field of
usefulness as the other iron coagulants. Because ferrous sulfate works better in feeding devices, compared with the ferric
coagulants, chlorinated copperas is sometimes preferred. The ferric hydroxide floc is heavier than alum floc and therefore
settles more rapidly.
( c ) Lime. Although lime is primarily used for pH control or chemical precipitation, it is also commonly used as a
co-coagulant.
8-3. Polyelectrolytes...
Polyelectrolytes are water-soluble organic polymers that are used as both primary coagulants and coagulant aids.
Polyelectrolytes are generally classified as follows :
• Anionic—ionize in solution to form negative sites along the polymer molecule.
• Cationic—ionize to form positive sites.
• Non-ionic—very slight ionization.
Polyelectrolyte primary coagulants are cationic, containing materials with relatively low-molecular weights (generally
less than 500,000). Cationic charge density (available positivecharged sites) is very high.
( a ) Coagulant aids, which are polyelectrolytes, may be anionic, cationic, or near-neutrally charged. Their molecular
weights are relatively high (range up to 20,000,000). They function primarily through interparticle bridging.
( b ) The efficiencies of polyelectrolyte primary coagulants depend greatly on the exact nature of the turbidity particles
to be coagulated, the amount of turbidity present, and the turbulence (mixing) available during coagulation.
8-4. Polyelectrolytes vs. Inorganic Coagulants...
Although they cannot be used exclusively, polyelectrolytes do possess several advantages over inorganic coagulants. These
are as follows :
• During clarification, the volume of sludge produced can be reduced by 50 to 90%.
• The resulting sludge is more easily dewatered and contains less water.
• Polymeric coagulants do not affect pH. Therefore, the need for an alkaline chemical such as lime, caustic, or soda ash
is reduced or eliminated.
• Polymeric coagulants do not add to the total dissolved solids concentration.
• Soluble iron or aluminum carryover in the clarifier effluent can result from inorganic coagulant use. By using polymeric
coagulants, this problem can be reduced or eliminated.
8-5. Coagulant Aids...
The coagulation process is often enhanced through the use of coagulant aids (or flocculants). Sometimes, excess primary
coagulant is added to promote large floc sizes and rapid settling rates. However, in some waters, even large doses of
primary coagulant will not produce a satisfactory floc. In these cases, a polymeric coagulant aid can be added after the
coagulant, to hasten reactions, to produce a denser floc, and thereby reducing the amount of primary coagulant required.
Because of polymer "bridging," small floc particles agglomerate rapidly into larger more cohesive floc, which settles
rapidly. Coagulant aids also help to create satisfactory coagulation over a broader pH range. Generally, the most effective
types of coagulant aids are slightly anionic polyacrylamides with very high-molecular weights. In some clarification
systems, non-ionic or cationic types have proven effective. The two types of coagulant aids discussed below are
synthetic-organic and natural-organic.
( a ) Synthetic Organic Coagulant/Coagulant Aids. Synthetic organic polymers are the most commonly used coagulant aids for
coagulation/flocculation of heavy metal precipitates (EPA, 1987). This is because metallic precipitates typically possess a
slight electrostatic positive charge resulting from charge density separation. The negatively charged reaction sites on the
anionic polyelectrolyte attract and adsorb the slightly positive charged precipitate (EPA, 1987). Synthetic organic
polyelectrolytes are commercially marketed in the form of dry powder, granules, beads, aqueous solutions, aqueous gels, and
oil-in-water emulsions (EPA, 1987). Generally, liquid systems are preferred because they require less floor space, reduce
labor requirements, and reduce the potential for side reactions because the concentrate can be diluted in the automatic
dispensing systems (EPA, 1987). Typical dosage requirements for metals-containing waters are in the 0.5- to 2.0-mg/L range.
Polyelectrolytes work most effectively at alkaline and intermediate pHs but lose effectiveness at pH levels lower than 4.5
(EPA, 1987).
( b ) Natural Organic Coagulant Aids. Coagulant aids derived from natural products include starch, starch derivatives,
proteins, and tannins (EPA, 1987). Of these, starch is the most widely used. The price per kilogram for these natural
products tends to be low; however, dosage requirements tend to be high (EPA, 1987). In addition, because of the composition
of natural products, they are more susceptible to microbiological attack, which can create storage problems.


