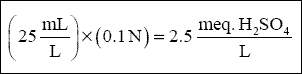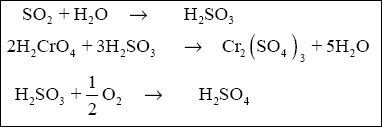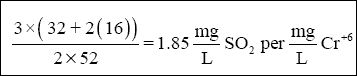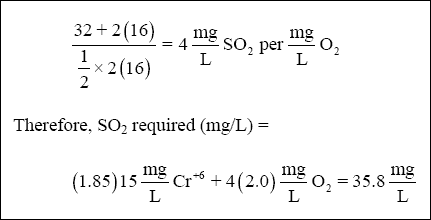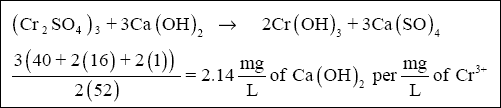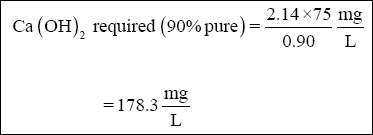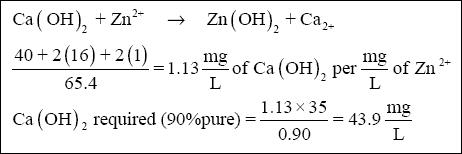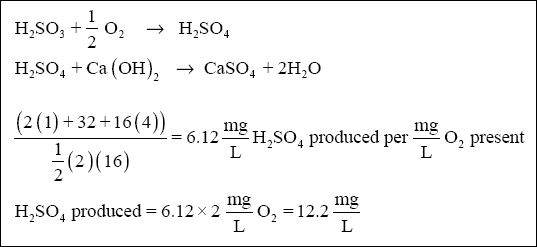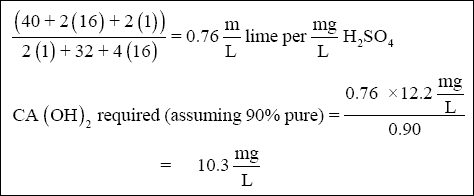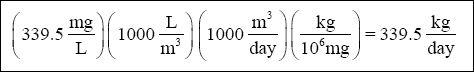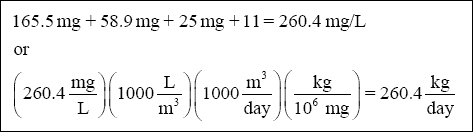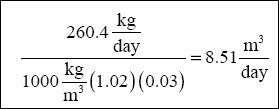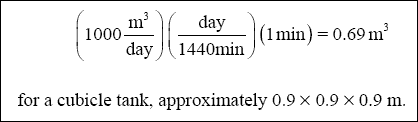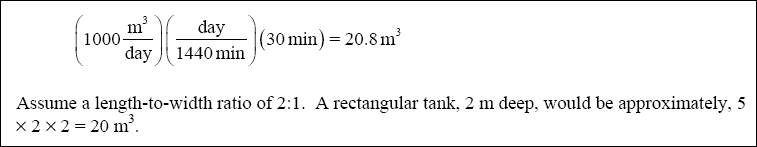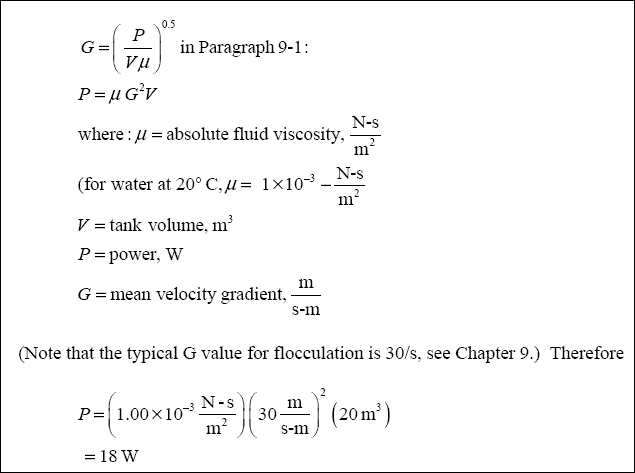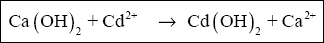Chemical Treatment - 14...
Chapter 14 - Design Examples...
Example - 1...
300,000 gal/day of landfill leachate and ground water have the following characteristics :
pH : 6.5.
Cr + 6 : 15 mg/L.
Total Cr : 75 mg/L.
Zn + 2 : 35 mg/L.
Suspended solids : 25 mg/L.
Dissolved oxygen : 2 mg/L.
( a ) The Feasibility Study determined that the method of treatment would be chemical reduction of chromium
(Cr + 6 to Cr + 3 ) using sulfur dioxide, followed by hydroxide precipitation using lime,
followed by coagulation, flocculation, and clarification. The full-scale treatment system will be designed to operate 24
hours / day.
( b ) During initial bench-scale testing, a 1-L sample of raw wastewater was titrated with 0.1 N sulfuric acid and then
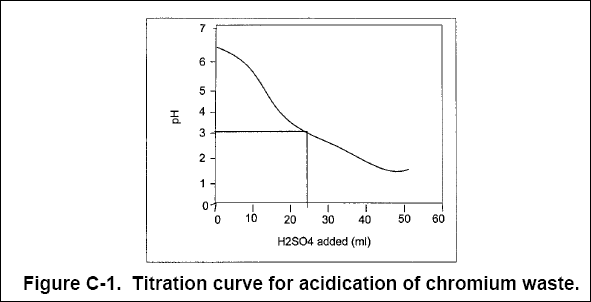
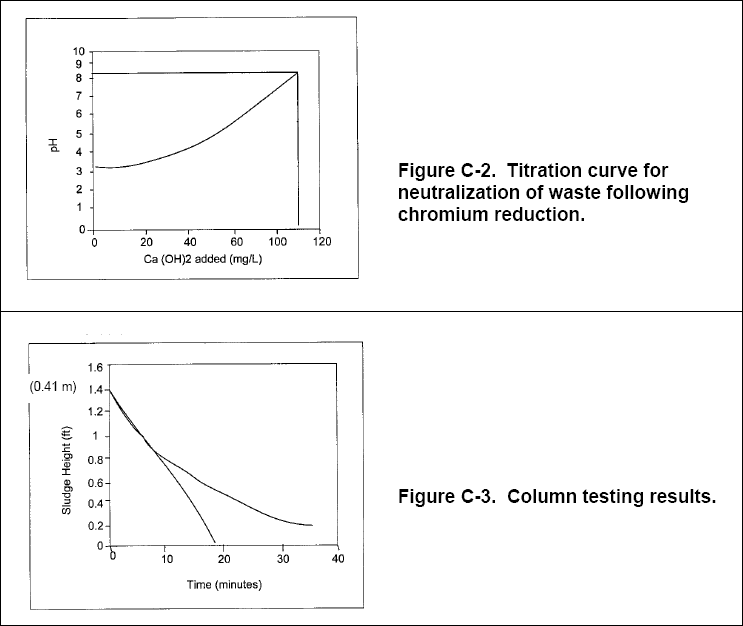
neutralized with lime, yielding the results shown in Figures C-1 and C-2 below.
( c ) Column testing was also conducted to determine the expected solids loading to the clarifier. Results of column
testing are shown in Figure C-3.
( d ) From the data given above, determine the following : (1) The chemical requirements to precipitate chromium and
neutralize the treated wastewater, assuming that sulfur dioxide will be used as the reducing agent and that lime will be
used as the precipitant. Assume that the final neutralized pH will be 8.5. (2) The daily sludge production, assuming that
10 mg/L ferric chloride will be used as a coagulant and that 1 mg/L polymer will be used as a coagulant aid. Assume that
the sludge has a specific gravity of 1.02 and is 3% solids. (3) The required coagulation and flocculation tank volumes,
using a 1-minute rapid-mix (coagulation) time and a 30-minute slow-mix (flocculation) time. (4) The theoretical power
requirement and required paddle area for the flocculation step, assuming a paddle tip speed of 1.2 ft/s. (5) The solids
settling area required for an inclined plate clarifier,assuming continuous treatment. Note the results of the column
testing shown in Figure C-3. (6) The effectiveness of the proposed method of treatment if the leachate originally
contained 5 mg/L of cadmium. (7) Additional testing to confirm the theoretical (stoichiometric calculations) results.
Solution - 1...
( a ) Chemical Requirements :
( 1 ) Sulfuric Acid. Sulfuric acid is required because chromium reduction using sulfur dioxide is typically conducted at a
pH of 2.5 to 3. Figure C-1 shows that approximately 25 mL of 0.1 N H 2 SO 4 is required to acidify
1 L of wastewater to pH 3.0. This is equal to an acid dose of :
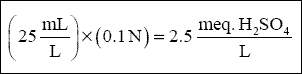
The acid feed rate required to achieve this dose will depend on the normality of acid used for treatment. (Note: Typical
required retention times for chromium reduction at a pH of 3 range from 2 to 20 minutes, depending on the initial
hexavalent chromium concentration.)
( 2 ) Sulfur Dioxide. The equations that govern the reduction of hexavalent chromium to trivalent chromium using sulfur
dioxide are as follows :
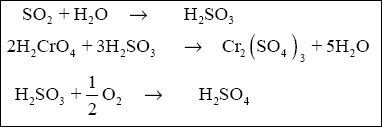
Using the above equations and molar ratios, and assuming that the reactions go to completion, gives the SO 2
requirements follows for Cr + 6 . Because 3 moles of SO 2 yield 3 moles of
H 2 SO 3 , then :
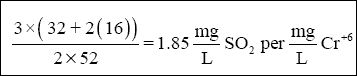
(Note that the quantity (32 + 2(16)), or 64, is the gram atomic -weight of sulfur dioxide. The quantity 52 is the gram
atomic-weight of chromium.) And the requirements for O 2 are :
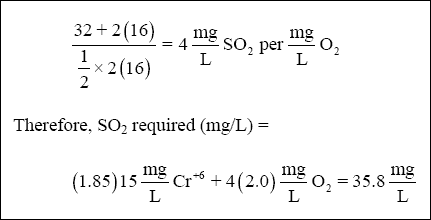
( 3 ) Lime. Lime is required to raise the pH of the wastewater to precipitate chromium. Assume that the lime is 90 % pure.
( a ) The amount of lime required to neutralize the wastewater to pH 8.5, as indicated in Figure 2-2, is approximately 107
mg/L.
( b ) The amount of lime required to precipitate the chromium can be calculated using the equation below :
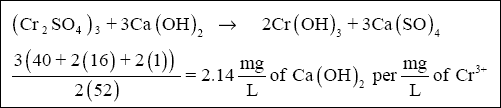
The total lime requirement to precipitate trivalent chromium is based on the combined total of trivalent and hexavalent
chromium present in the raw wastewater (75 mg/L).
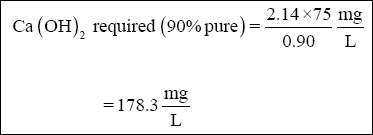
( c ) Lime is also required to precipitate zinc (35 mg/L). The minimum solubility of zinc hydroxide occurs at approximately
pH 9.0 (relatively close to 8.5see Figure 2-2). Therefore, for simplicity, assume that the lime reaction with zinc goes to
completion at pH 8.5. The amount of lime can be calculated using the equation below :
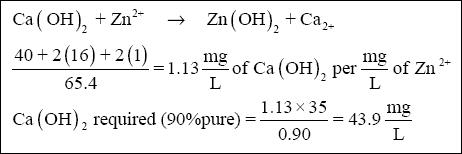
( d ) The amount of lime required to neutralize the H 2 SO 4 produced from dissolved oxygen
initially present in the wastewater can be calculated from the equations below :
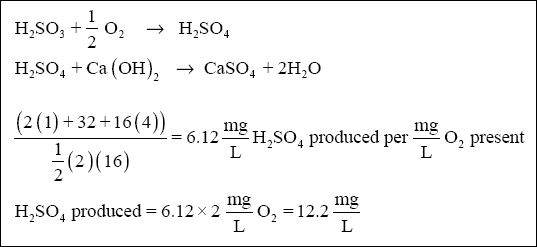
Therefore, the lime requirement, from the second equation, is :
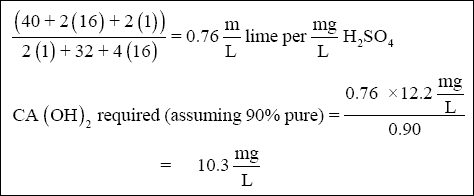
( e ) Therefore, the total amount of lime = 107 + 178.3 + 43.9 + 10.3 = 339.5 mg/L
Or, in kg / day :
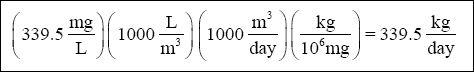
( b ) Daily Sludge Production :
( 1 ) Chromium hydroxide sludge :
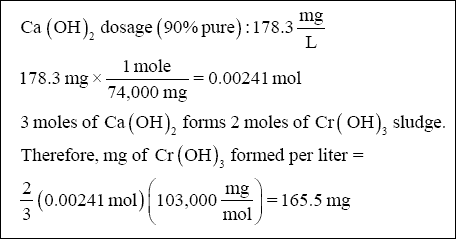
( 2 ) Zinc hydroxide sludge :
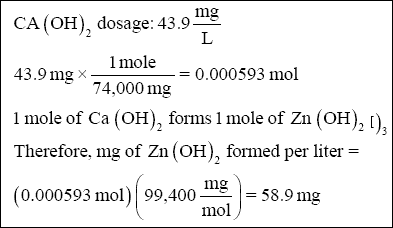
( 3 ) Suspended solids : Given in problem statement (25 mg/L).
( 4 ) Coagulant and coagulant aid : Given in problem statement. Assume that all coagulant and coagulant aid settle out of
solution and subsequently contribute to the sludge volume. Therefore 10 mg/L of coagulant + 1 mg/L of coagulant aid = 11
mg/L.
( 5 ) Total sludge :
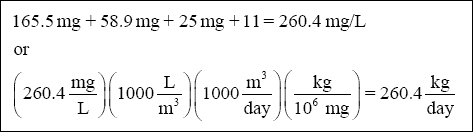
As mentioned in the problem statement, with the assumption that the sludge is 3% solids and that the specific gravity of
the sludge is 1.02, the volume that will require disposal each day can be calculated as follows :
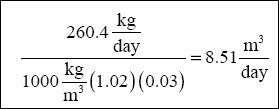
( c ) Required Volumes of Coagulation and Flocculation Units :
( 1 ) Coagulation Tank :
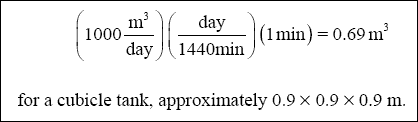
( 2 ) Flocculation Tank :
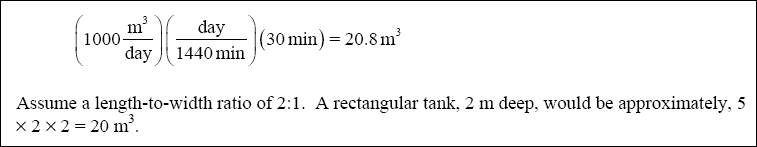
( d ) Calculate the Theoretical Power Requirement and Required Paddle Area for the Flocculation Step :
( 1 ) Theoretical Power Requirement : Rearranging the equation :
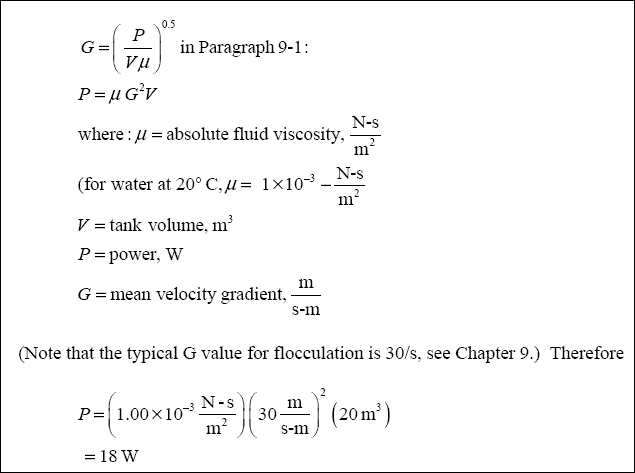
( 2 ) Paddle Area Requirement : Rearranging the equation ;

( e ) Calculate the Solids Settling Area Required for Inclined Plate Clarifier :
Figure C-3 shows the results of the column testing. The column tests determined that the settling rate was 0.41 m in 18
minutes. Convert this to commonly used units (Note: 0.41 m is the height of the solid/liquid interface at time zero, when
the column is filled to 1,000 mL) :
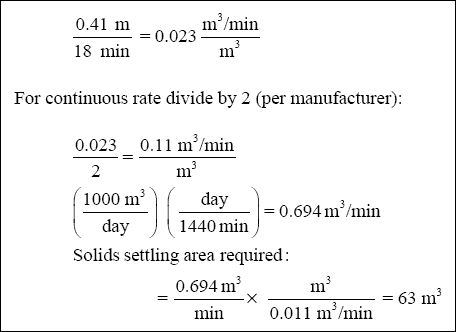
( Note : Most manufacturers of inclined plate clarifiers recommend a rate of 0.010 (m 3 / min) / m 2
for metal hydroxide precipitates. Rates should not exceed 0.04 (m 3 / min) / m 2 . )
( f ) How Effective Would the Proposed Method of Treatment be if the Leachate Originally Contained 5 mg/L of
Cadmium ? :
See Figure 2-2. Cadmium is removed through hydroxide precipitation as follows :
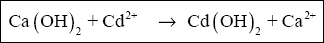
Note that the minimum solubility of cadmium hydroxide occurs at pH > 11. At a pH of 8.5 the solubility of cadmium hydroxide
is high (greater than 100 mg/L). Therefore, by raising the pH to only 8.5 or 9 (to remove chromium and zinc), the cadmium
concentration would not be reduced below the original concentration of 5 mg/L. To effectively remove cadmium, a second
stage precipitation/clarification step would be required where the pH would be raised to 11.
( Note : Results of jar testing could show that co-precipitation of cadmium hydroxide may occur, thereby effectively
lowering the cadmium hydroxide concentration.)
( g ) Should Additional Tests be Conducted to Confirm the Theoretical Results ? :
Yes, it is important that jar testing be conducted to determine if the theoretical results are accurate. Oftentimes,
actual chemical requirements can differ significantly with stoichiometric calculations. This can be caused by a number of
things (see Paragraph 2-2).