| Type of sludge | SVI ( mL / g ) | ZSV at 3.5 g / L ( m / h ) |
| Well settling | < 100 | > 3 |
| Light | 100 - 200 | 2 - 3 |
| Bulking | > 200 | < 1.2 |
| Time ( minute ) | SSV ( mL / L ) |
| 0 | 1,000 |
| 5 | 500 |
| 10 | 400 |
| 15 | 325 |
| 20 | 290 |
| 25 | 260 |
| 30 | 250 |
| 40 | 220 |
| 50 | 200 |
| 60 | 200 |
 "Graphical Representation of the Results Obtained from Settleometer Test"...
"Graphical Representation of the Results Obtained from Settleometer Test"...
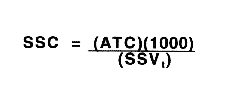
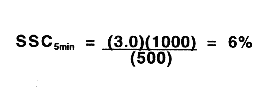
| Time ( minute ) | SSV ( mL / L ) | SSC ( % ) |
| 0 | 1,000 | 3.0 |
| 5 | 500 | 6.0 |
| 10 | 400 | 7.5 |
| 15 | 325 | 9.2 |
| 20 | 290 | 10.3 |
| 25 | 260 | 11.5 |
| 30 | 250 | 12.0 |
| 40 | 220 | 13.6 |
| 50 | 200 | 15.0 |
| 60 | 200 | 15.0 |
 "The Graph of SSV and SSC"...
"The Graph of SSV and SSC"...
 "The Graph of RSC Value on the Graph"...
"The Graph of RSC Value on the Graph"...
 "More Settleometer Curve Examples"...
"More Settleometer Curve Examples"...
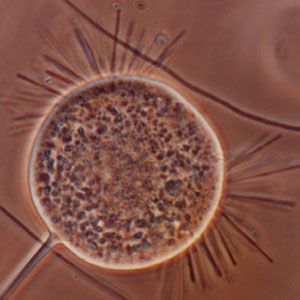
| Numerical value | Abundance | Explanation |
| 0 | None | - |
| 1 | Few | Filaments present, but only observed in an occasional floc |
| 2 | Some | Filaments commonly observed, but not present in all flocs |
| 3 | Common | Filaments observed in all flocs, but at low density (e.g. 1 to 5 filaments per floc) |
| 4 | Very common | Filaments observed in all flocs at medium density (e.g. 5 - 20 per floc) |
| 5 | Abundant | Filaments observed in all flocs at high density (e.g. 20 per floc) |
| 6 | Excessive | They present in all flocs, appears more filaments than flocs and / or filaments growing in high abundance in bulk solution |
| SI ( % ) | The extent of problems |
| 0 - 0.5 | Negligible |
| 0.5 - 6 | Small |
| 6 - 10 | Medium |
| 10 - 15 | Serious |
| > 15 | Catastrophic |